BAYOOSOFT Risk Manager
The efficient route to technical documentation
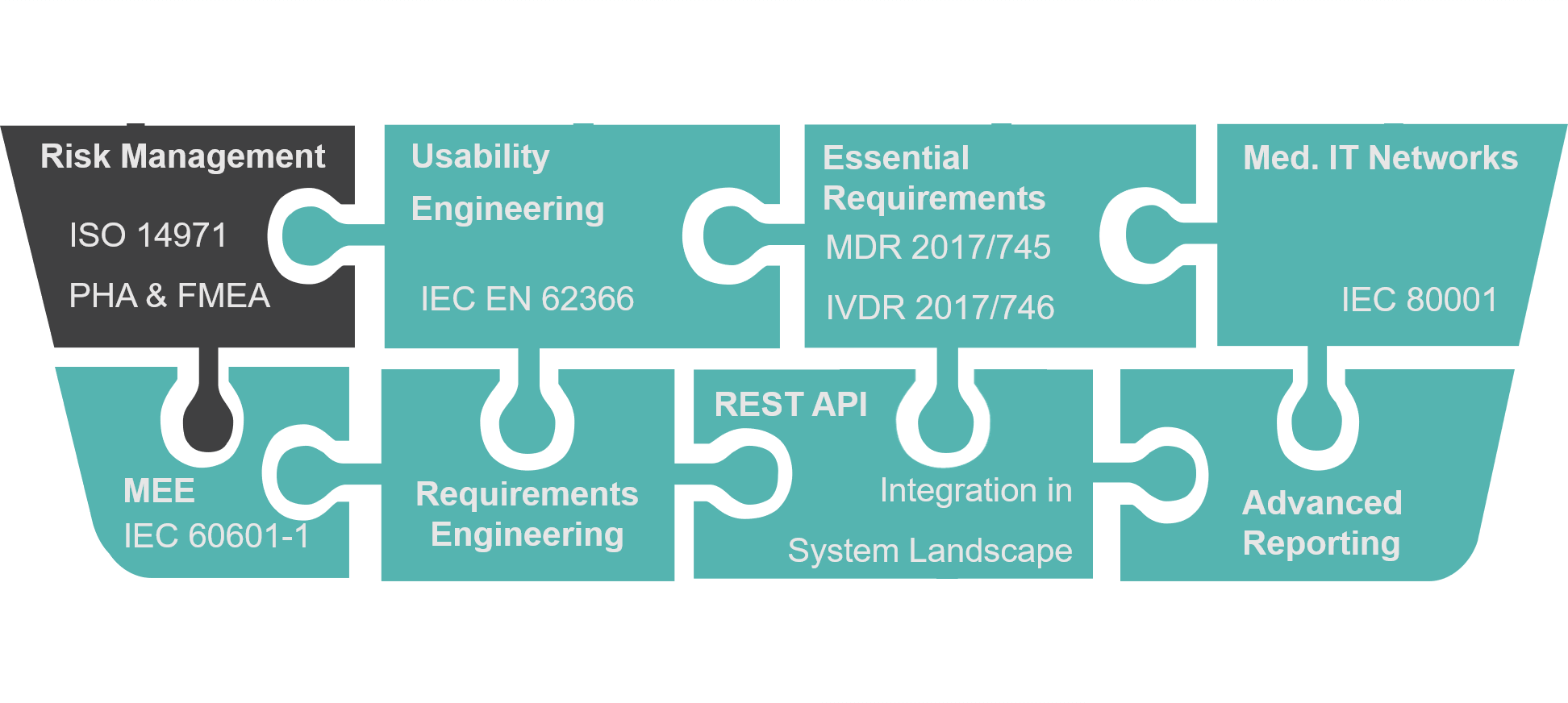
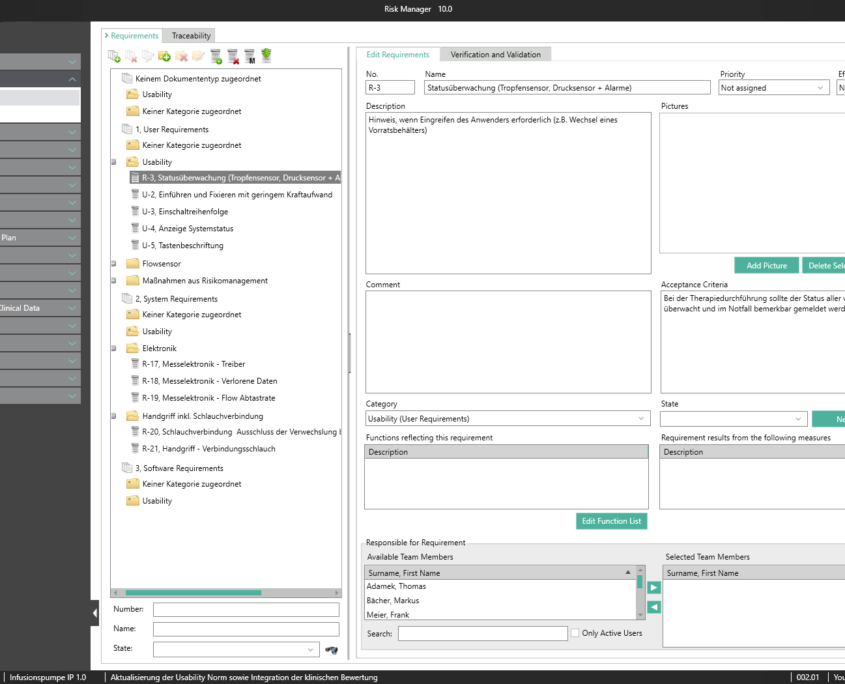
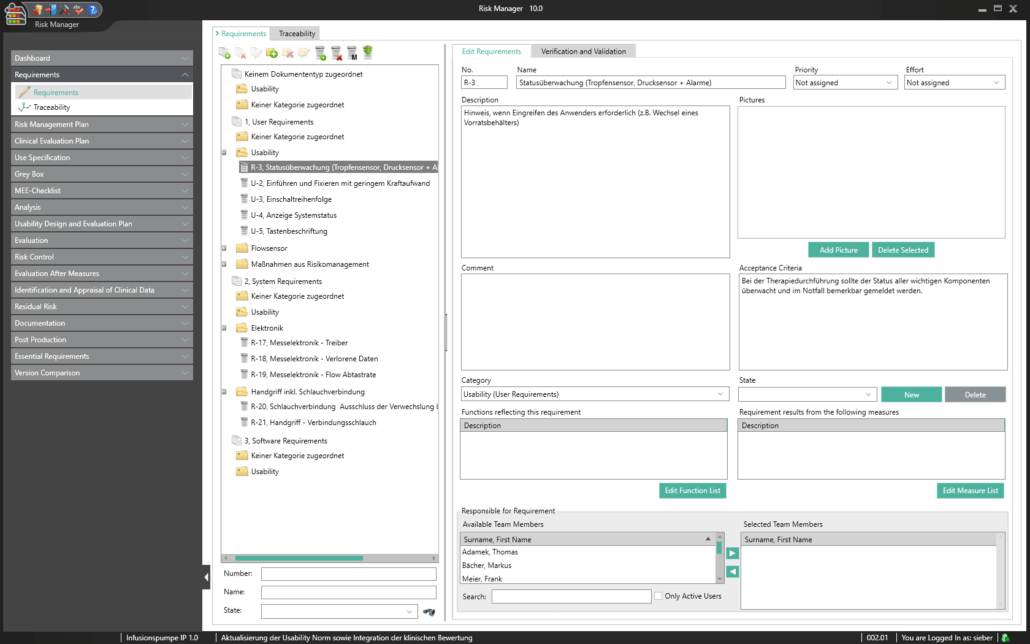
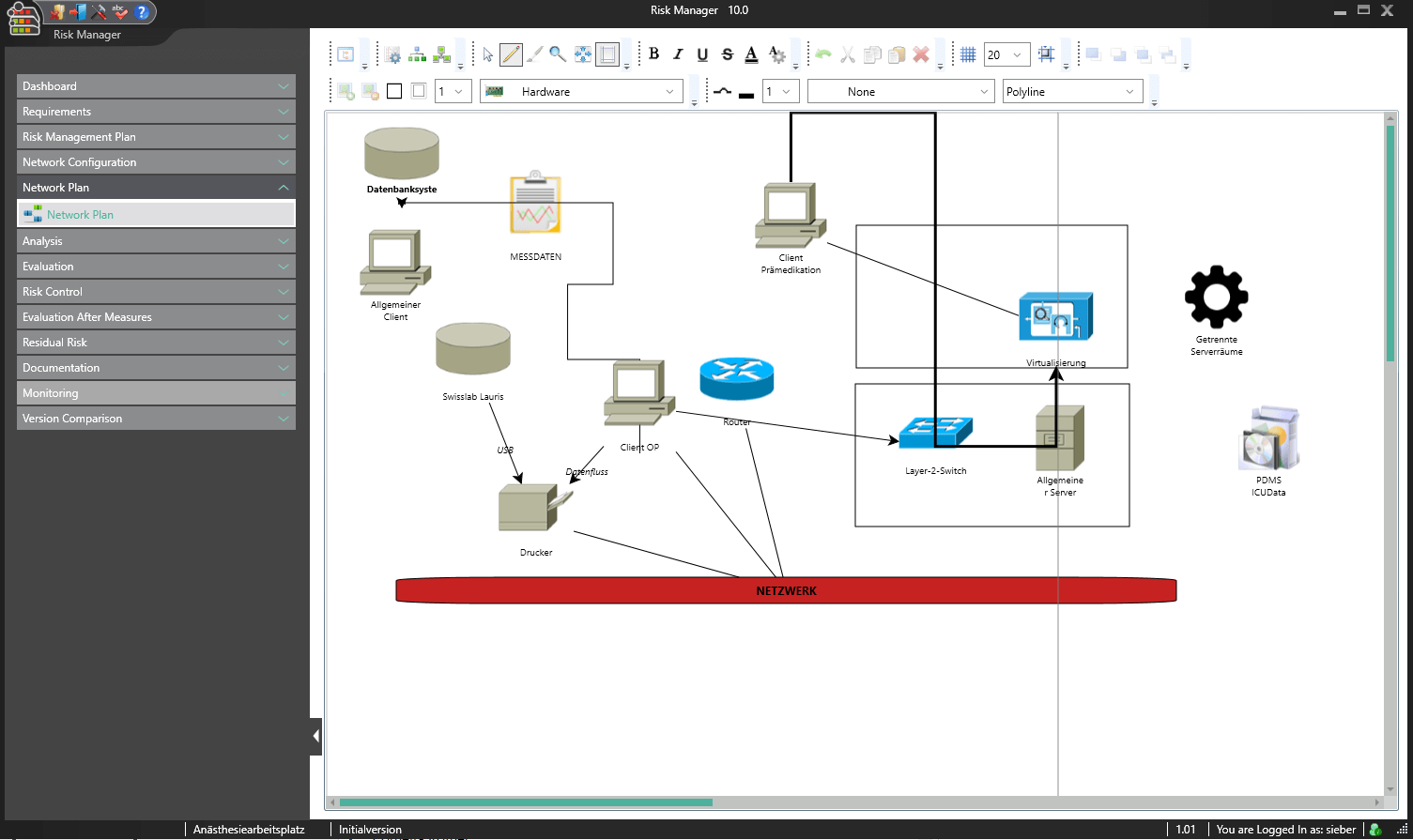
BAYOOSOFT Risk Manager is the validated approval accelerator for conveniently generating auditable technical documentation for medical devices. The globally leading medical device manufacturer has been providing support with a process-oriented approach in the preparation of risk management documents according to ISO 14971, in usability engineering according to IEC 62366, the creation of conformity reports according to IEC 60601-1 as well as the fulfillment of the essential requirements of MDD, IVDD, MDR and IVDR since 2000. At the same time, BAYOOSOFT Risk Manager includes fully integrated requirements engineering, which significantly simplifies the traceability of requirements. For clinics and hospitals, we offer an extension module for medical IT networks according to IEC 80001-1. In order to provide even greater support in the preparation of technical documentation, the extension modules for Clinical Evaluation and support in the software lifecycle process according to IEC 62304 are also available.
Validated as category-four software according to GAMP 5, BAYOOSOFT Risk Manager offers a Pre-Validation Package for support with system validation required in your environment. Advanced Reporting as well as a REST API are also available for customization and technical integration into the existing system landscape.
Learn more about BAYOOSOFT Risk Manager modules or arrange a consultation for a product presentation straight away. We will be happy to advise you.
RISK MANAGEMENT ACCORDING TO DIN EN ISO 14971
The internationally valid standard ISO 14971 stipulates a procedure which identifies, assesses, rates and controls hazards associated with medical devices and their accessories, and monitors the effectiveness of these controls. The requirements of this standard on risk management are fully integrated in BAYOOSOFT Risk Manager for preparing compliant risk management documents. The procedure follows a clearly structured approach based on the process steps of the standard:
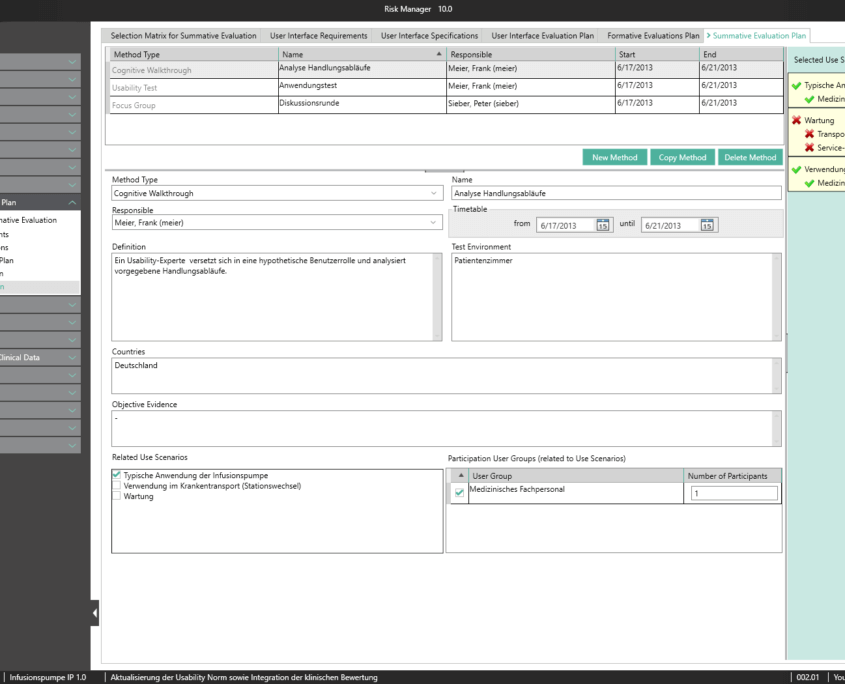
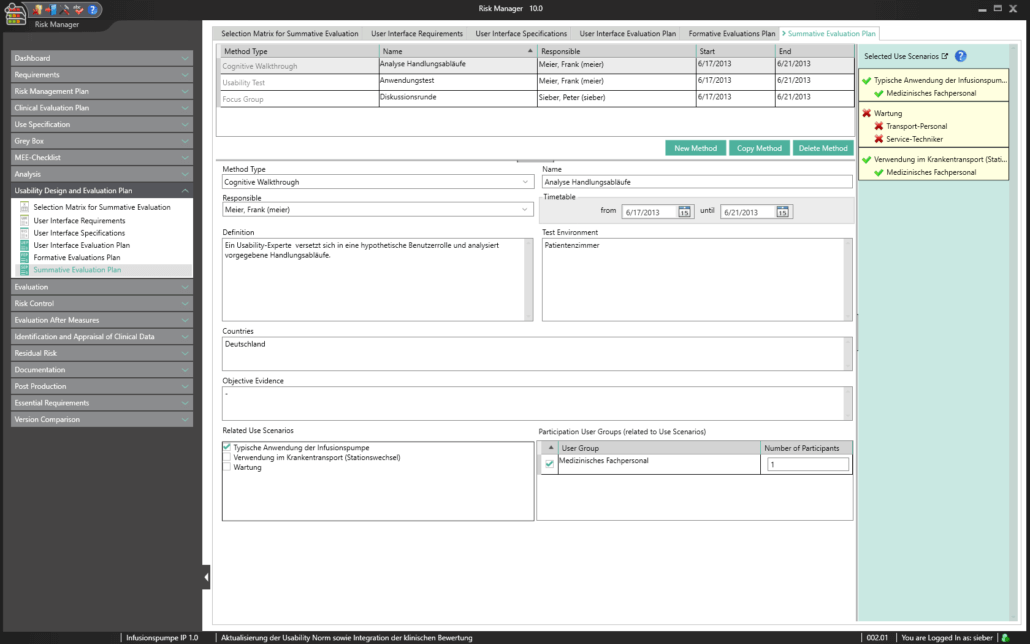
Here, BAYOOSOFT Risk Manager offers the choice between two risk analysis methods:
In both cases, you can define global risk templates and set your own individual risk definitions in each project, and even in each version.
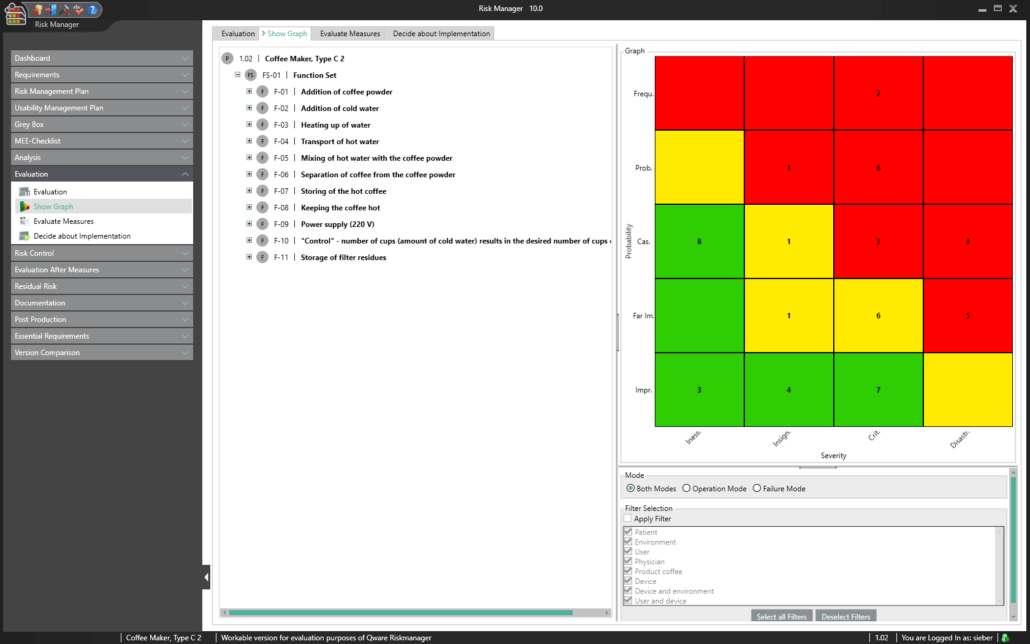
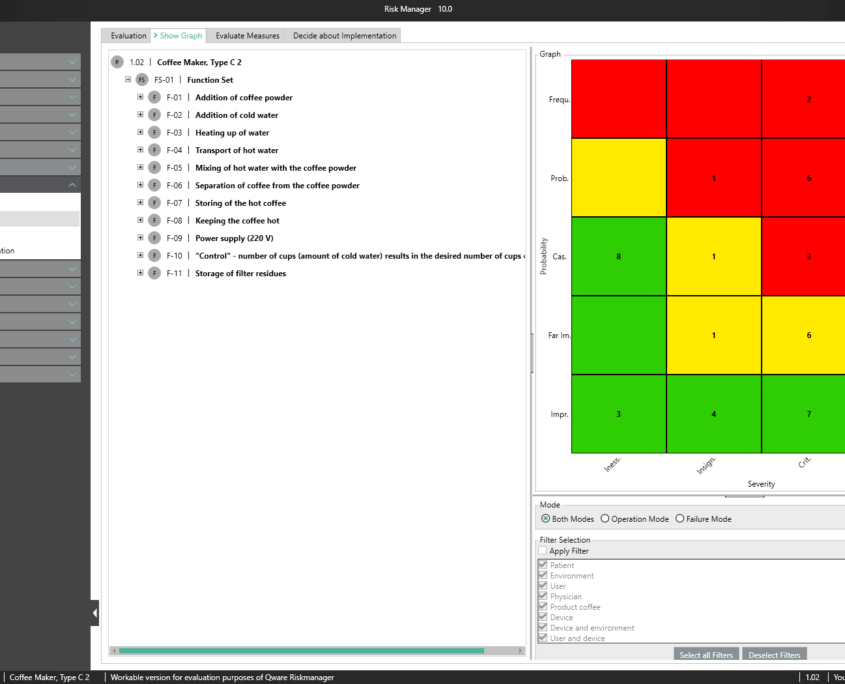
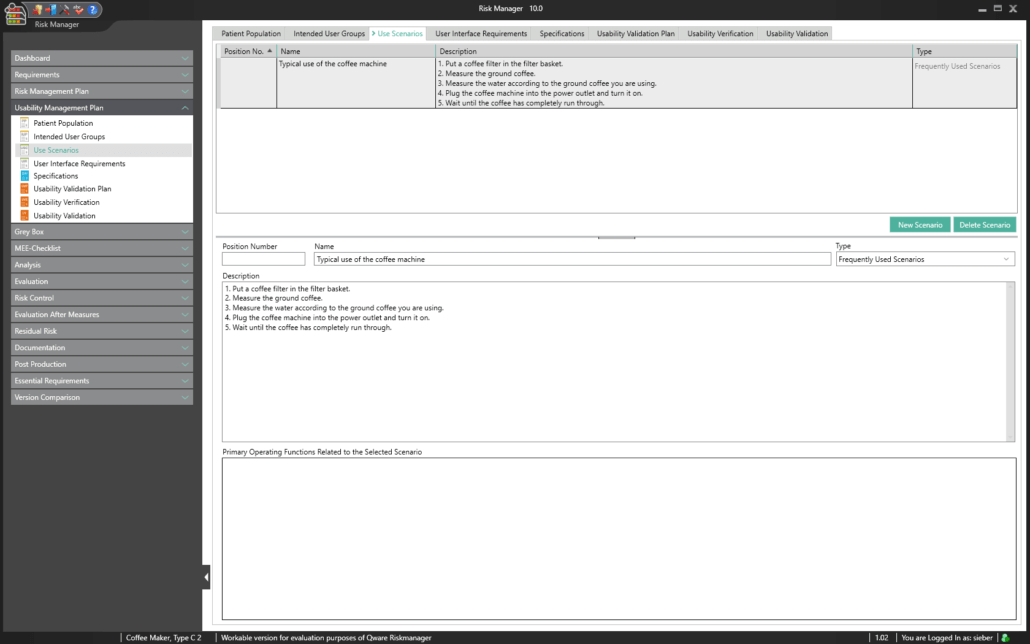
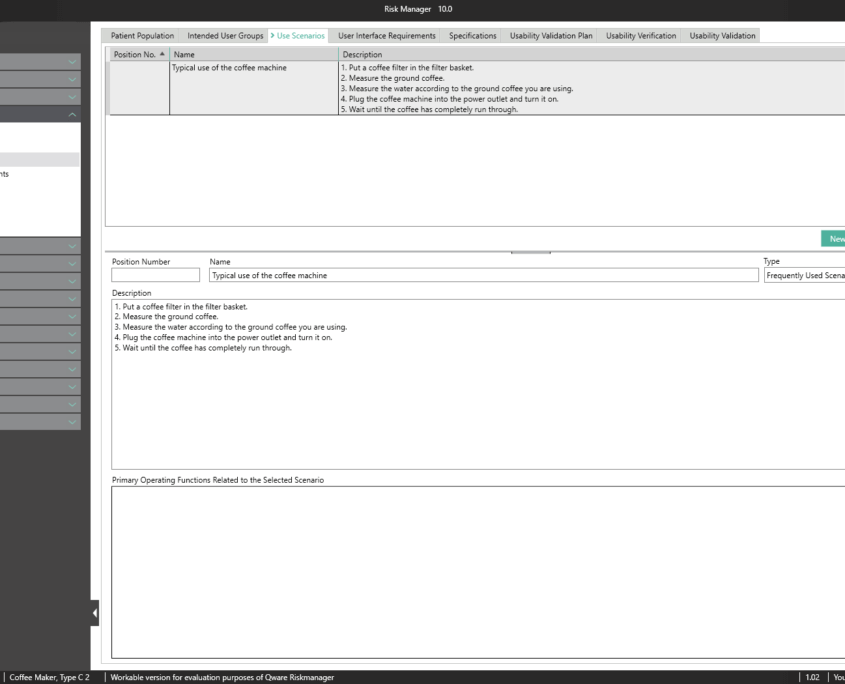
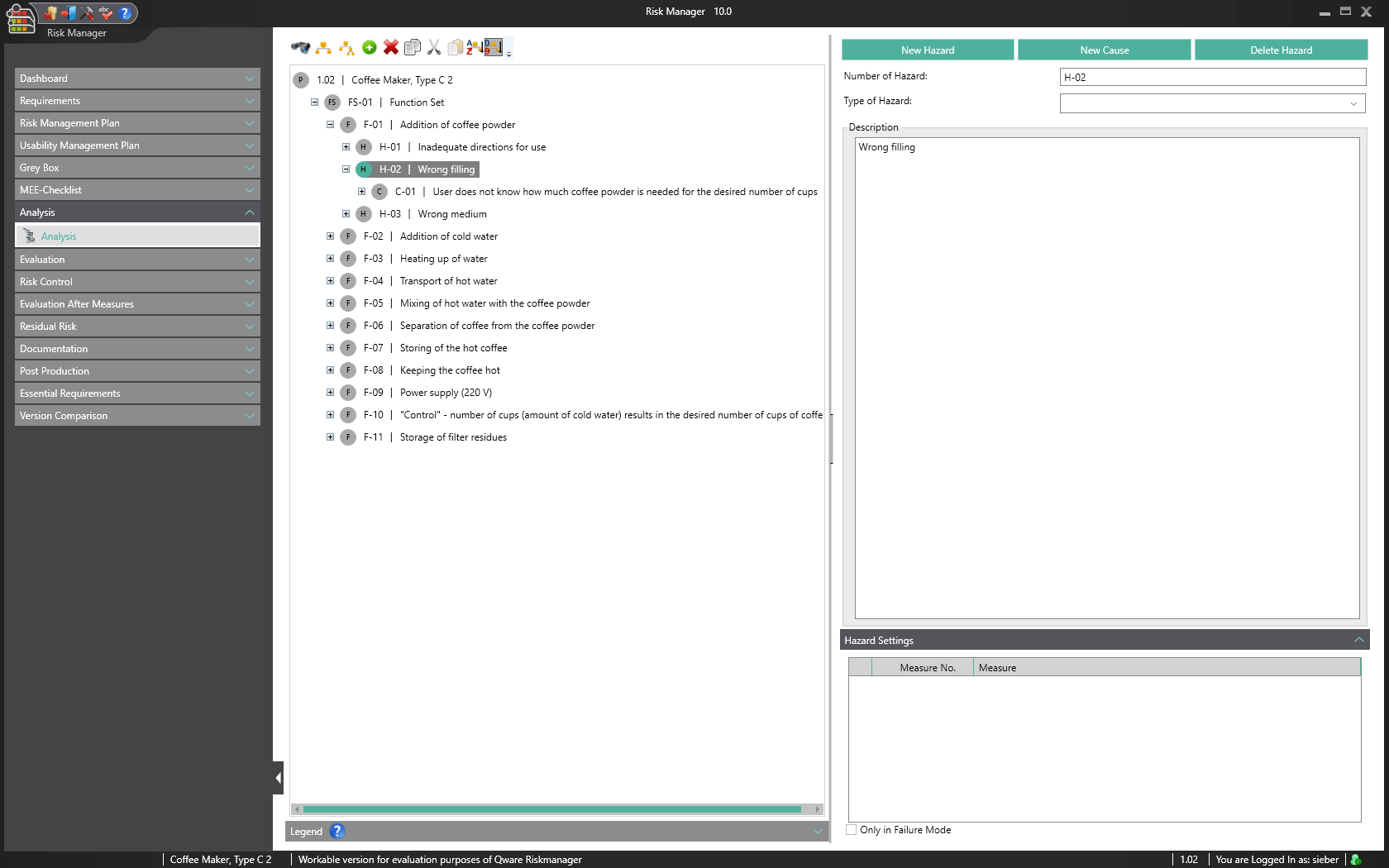
The central component of BAYOOSOFT Risk Manager is the risk management tree, comprising features, hazards, causes and measures with the preliminary hazard analysis (PHA) method.
In the case of the FMEA method, these are functions, failure type, failure effect, failure cause as well as the current and recommended measures.
With the risk analysis, you can build on your existing knowledge by copying or importing parts of the tree or a complete risk analysis, or by interactively using the knowledge database. Moreover, powerful tools are available including a content-dependent search-and-replace feature that – unlike Office applications – ensures that replacements are only implemented at the intended positions within your documentation.
1
2
1
Structured procedure in a tree view
2
Description and categorisation of hazards
Depending on the chosen method (FMEA or PHA), the risks are assessed at the level of causes or based on the combination of failure type, failure effect and failure cause. The risk graph or FMEA diagram is updated even while entering the assessment. This means you can instantly see the current assessment status for the respective measure through to the complete component, product or process.
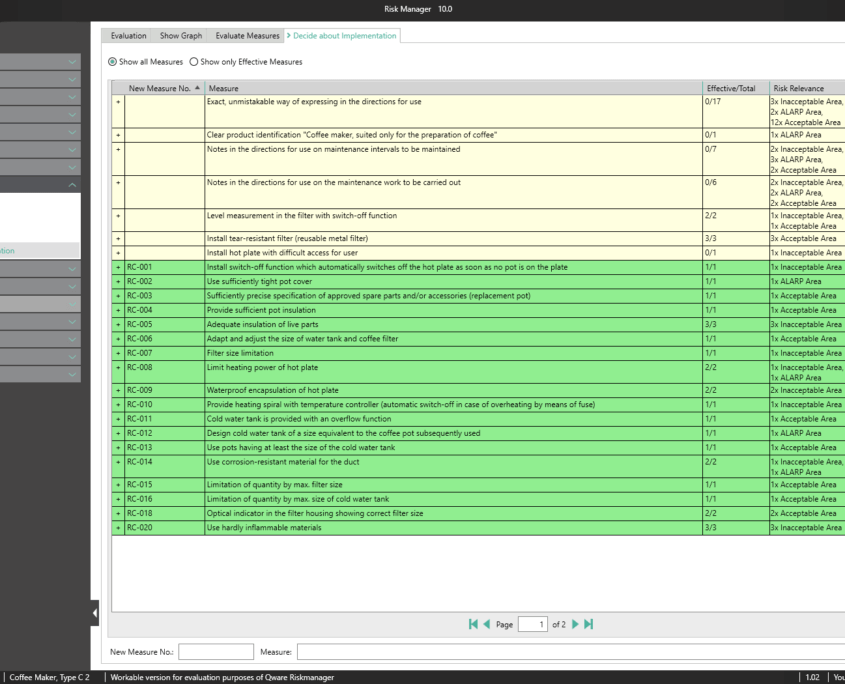
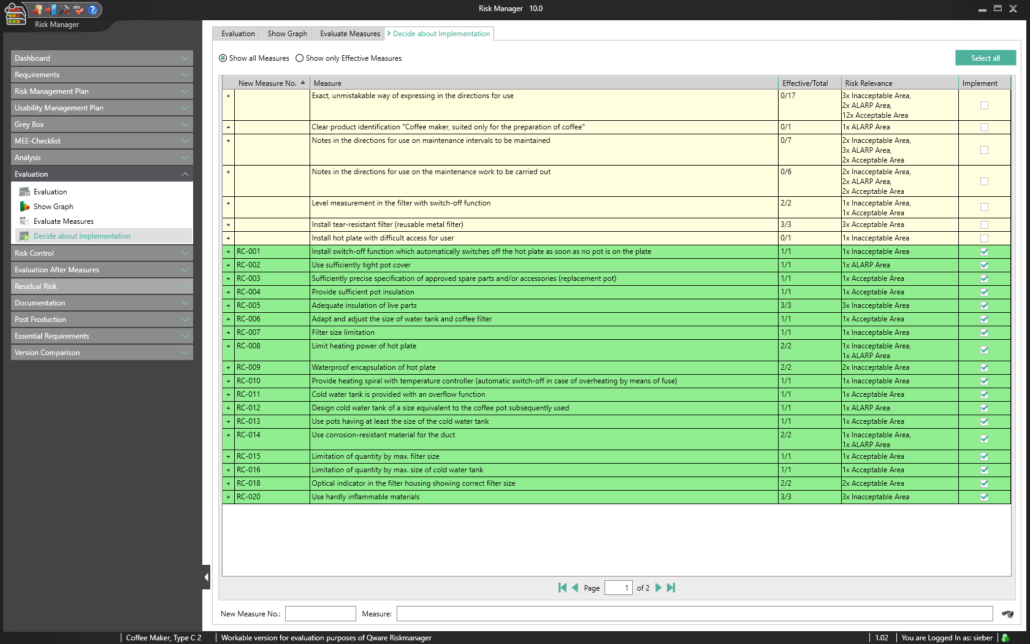
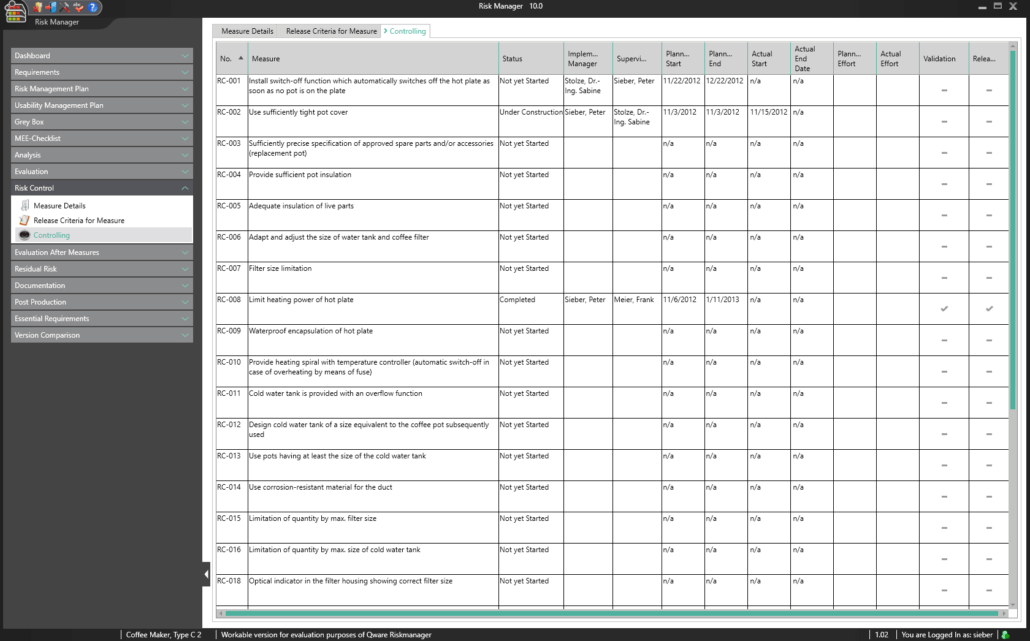
BAYOOSOFT Risk Manager automatically calculates the efficiency of the risk-reducing measures you take and summarizes them in a clear list of results. This way, you can see at a glance which measures would reduce multiple risks at once and which measures reduce risks that lie outside the acceptable range, for example. The user selects the measures with the click of the mouse, which starts the process of measure management.
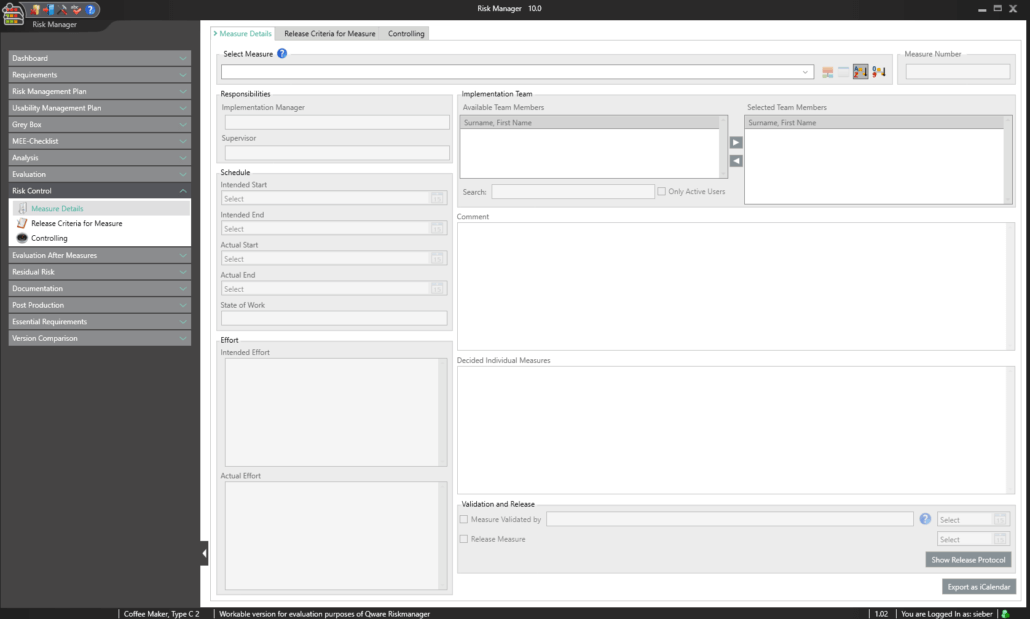
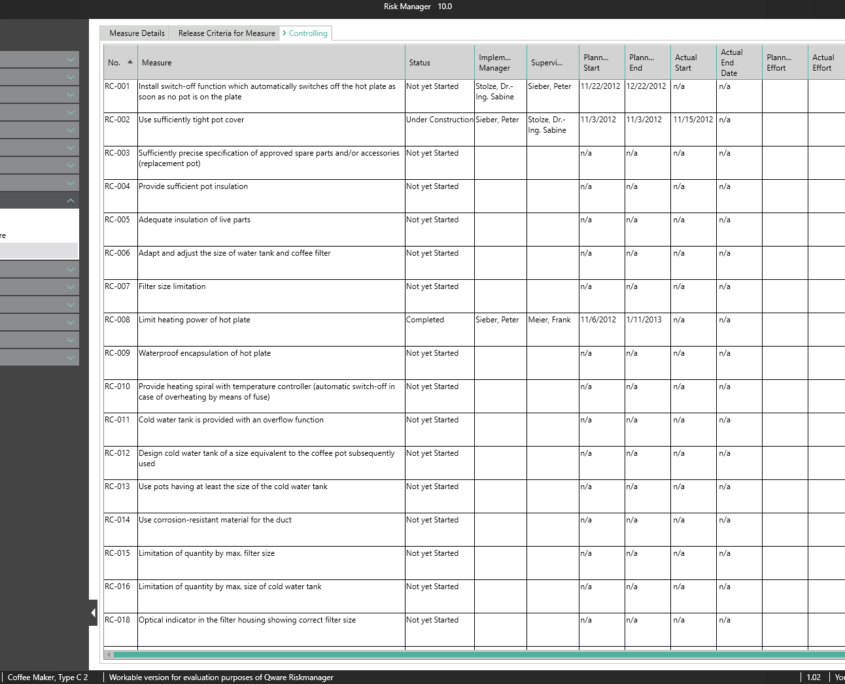
A key part of the technical documentation is the process of measure management. BAYOOSOFT Risk Manager automatically calculates the efficiency of the risk-reducing measures taken and summarizes them in a clear list of results. This way, you can see at a glance which measures reduce multiple risks at once and which measures reduce risks that lie outside the acceptable range, for example. For each selected measure, you can:
- Define responsibilities (implementation and monitoring controllers)
- Compose implementation teams
- Add schedules and cost plans
- Define individual clearance criteria (e.g. process for proving effectiveness, process for verification and validation, acceptance limits)
A controlling overview provides a summary of the current progress for all measures adopted.
When reassessing the risks, BAYOOSOFT Risk Manager checks whether risks were reduced without corresponding risk-reducing measures being implemented, and indicates this potential error to the user. If new hazards arise from the measures implemented, corresponding relationships can be established.
The process is completed consistently by allowing new requirements to be generated from adopted measures at the press of a button.
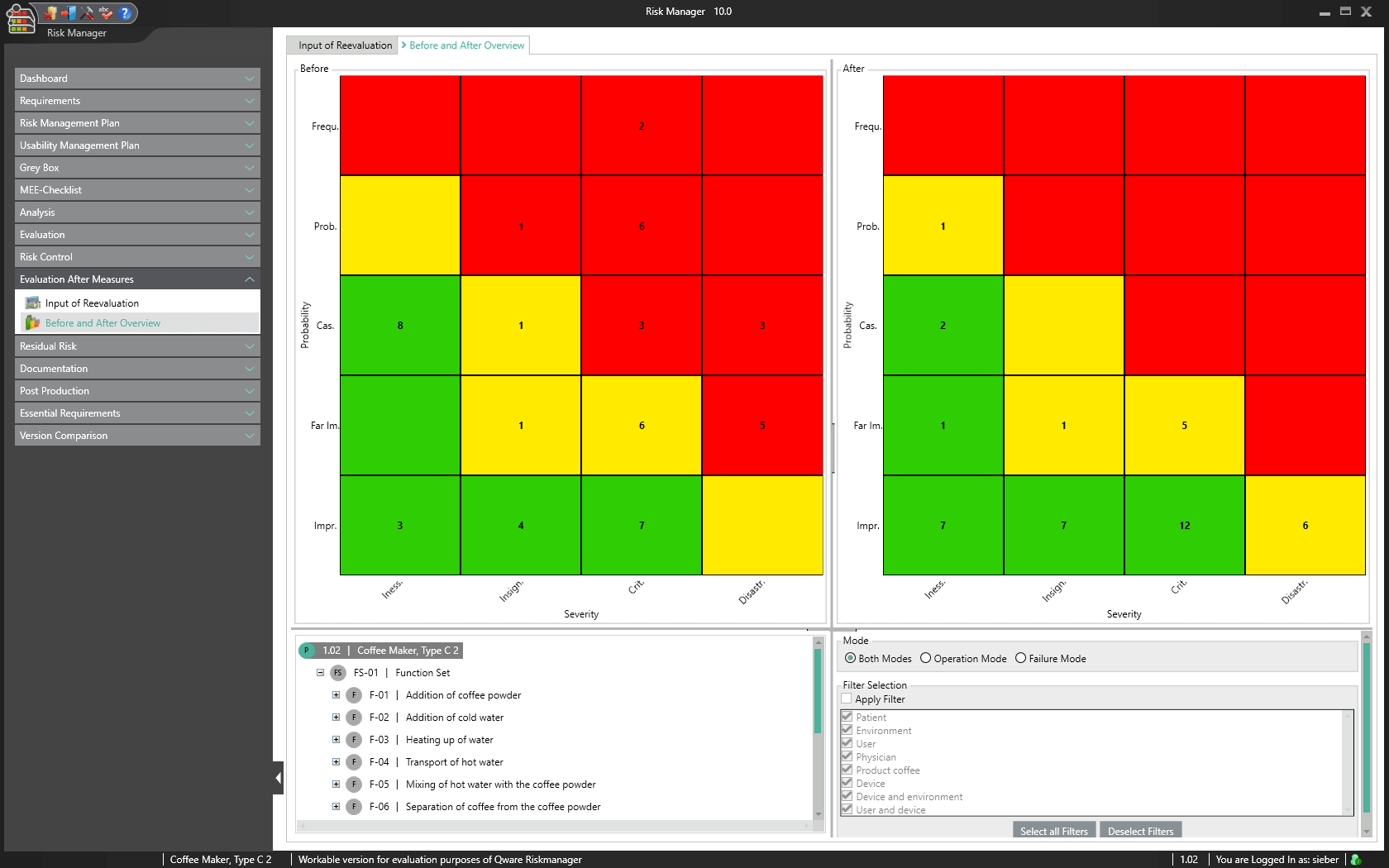
Risks are reassessed as part of a re-evaluation following implementation of the measures. The user can see in a risk graph which risks remain in which areas and can directly read out the effectiveness of risk reduction based on the before/after overview. Interactive features guide the user directly to the points that do not meet the desired acceptance criteria and supports the user with the help of the self-learning knowledge database with suggestions that may lead to further risk reduction. Automatic checks support the user by assessing and reassessing all risks and helps avoid evaluation errors.
As a manufacturer of medical devices, you are subject to a vigilance system according to European medical devices law, which encompasses all levels of market monitoring for the medical devices you bring into circulation. This includes monitoring and assessing all incidents and corresponding hazard prevention, such as recalls. BAYOOSOFT Risk Manager offers you a dedicated area for market monitoring, allowing you to document your observations. If during market monitoring you determine that a new risk assessment is necessary, this can likewise be documented. The integrated reminder system lets you add BAYOOSOFT Risk Manager reminders to Microsoft Outlook or Lotus Notes, supporting you in regular market monitoring for the given product.
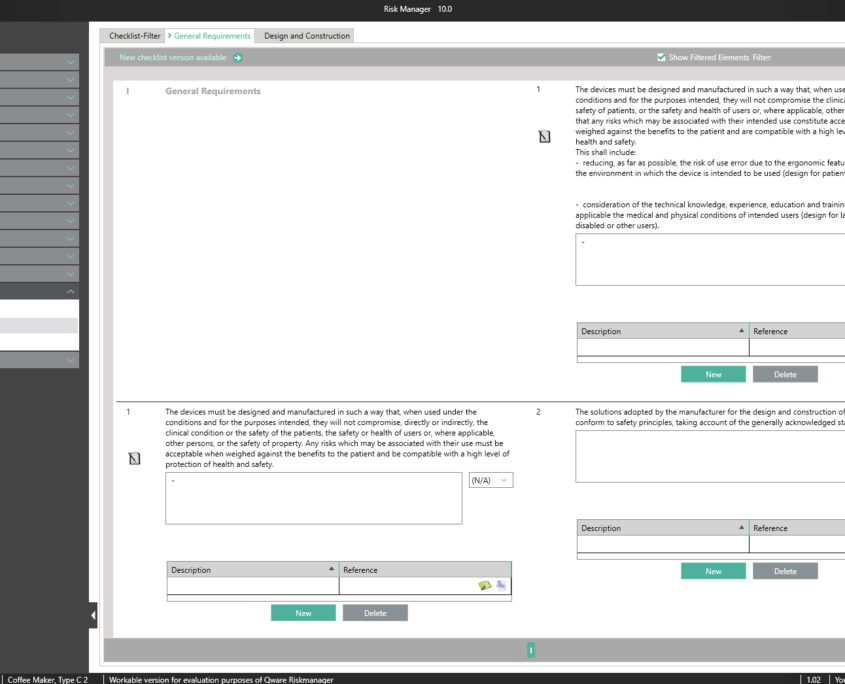
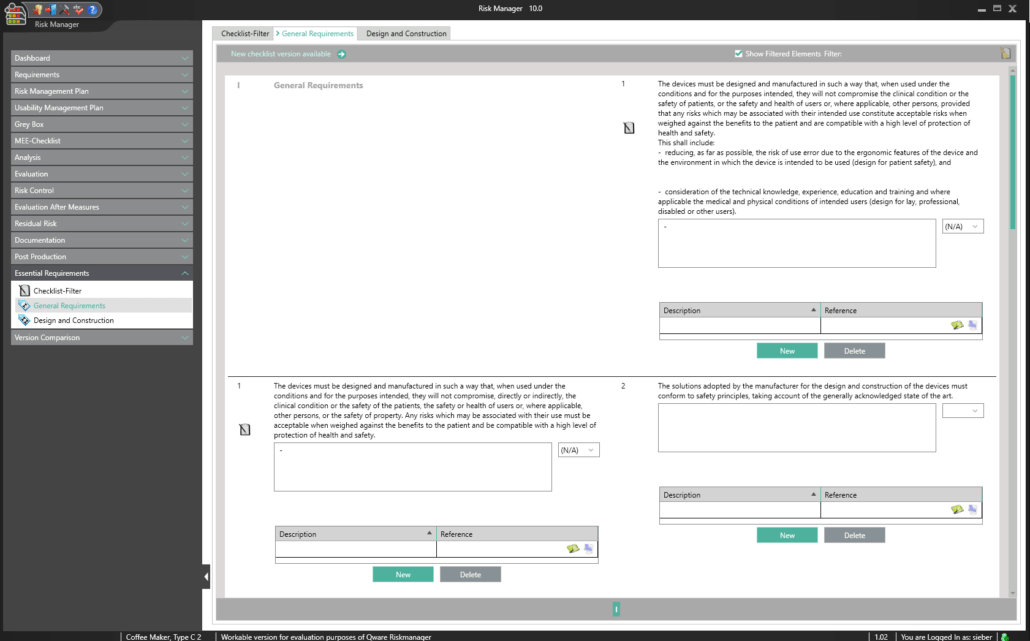
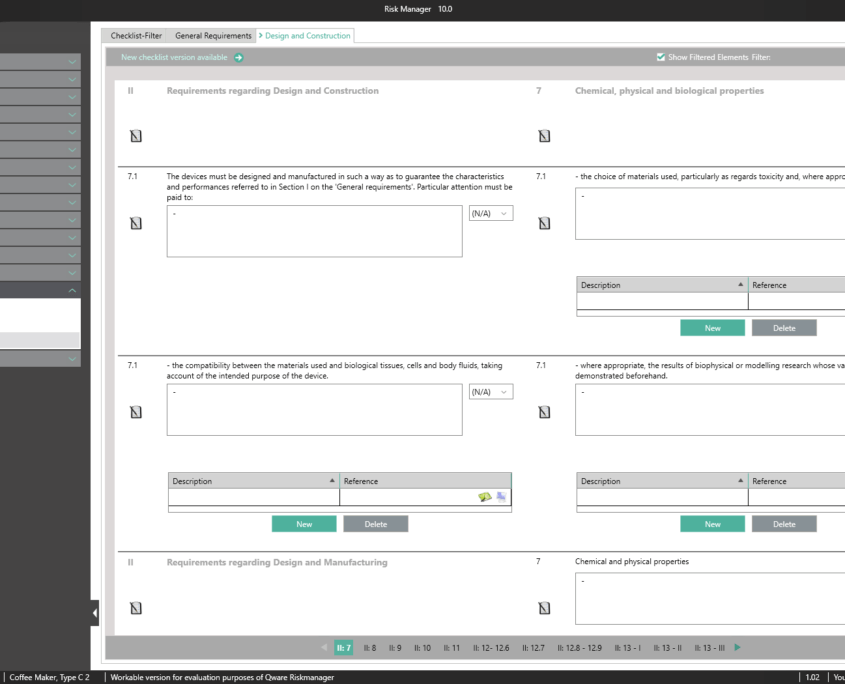
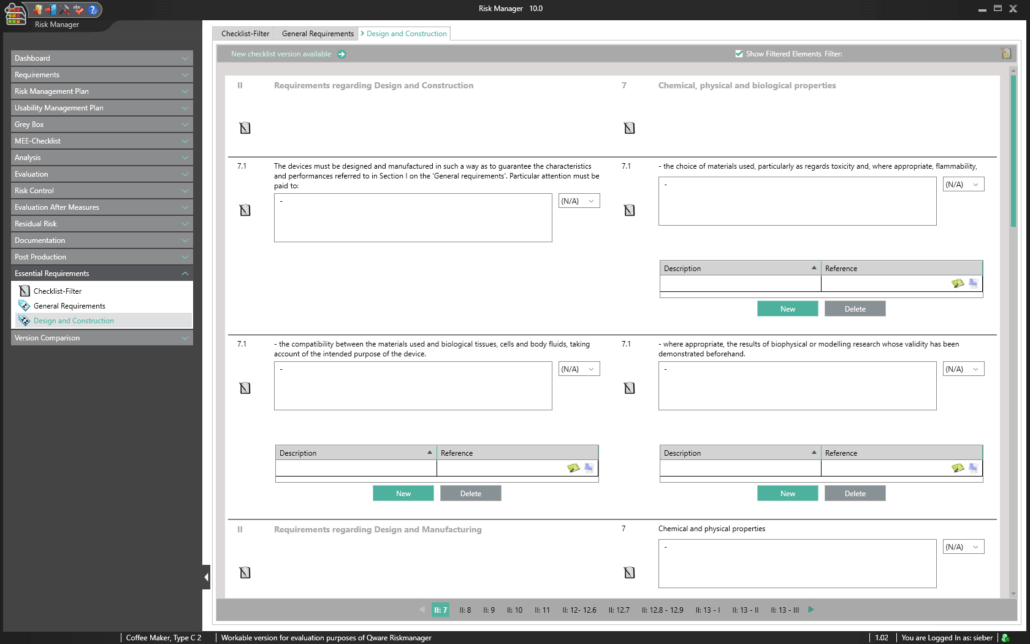
Essential Requirements According to MDR 2017/745 & IVDR 2017/746
To obtain a CE marking for your medical device and hence approval for distribution in Europe, it’s necessary to comply with essential requirements as well as a conformity assessment process prescribed for the respective medical device. In the case of in-vitro diagnostics, the essential requirements are stipulated in IVDR 2017/746, and for other medical devices in MDR 2017/745.
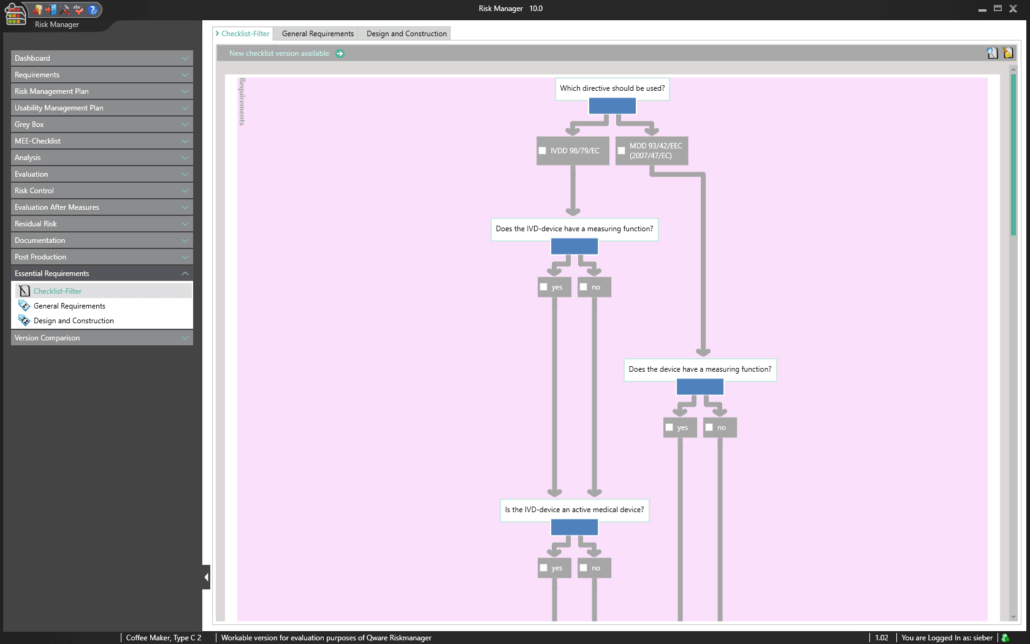
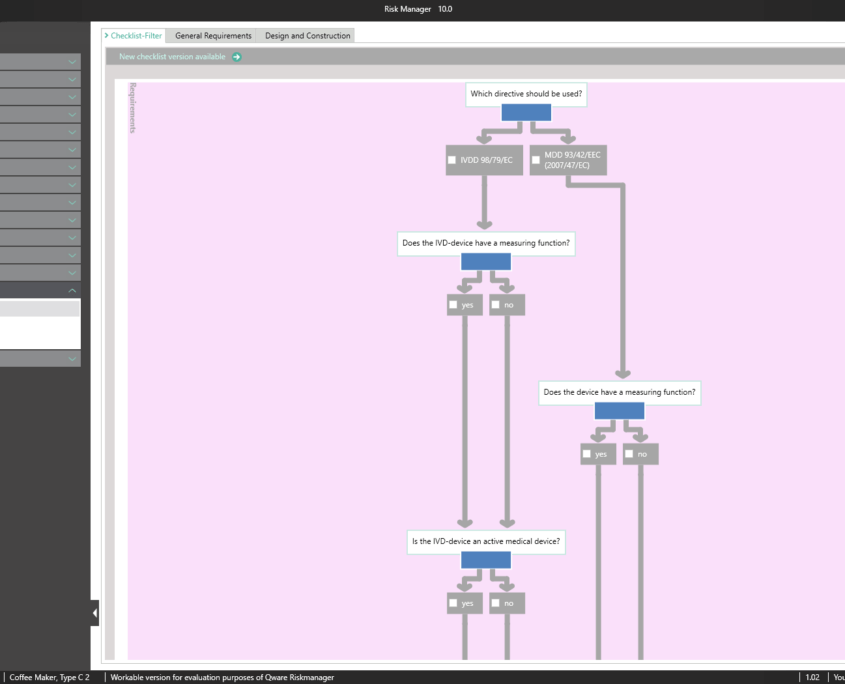
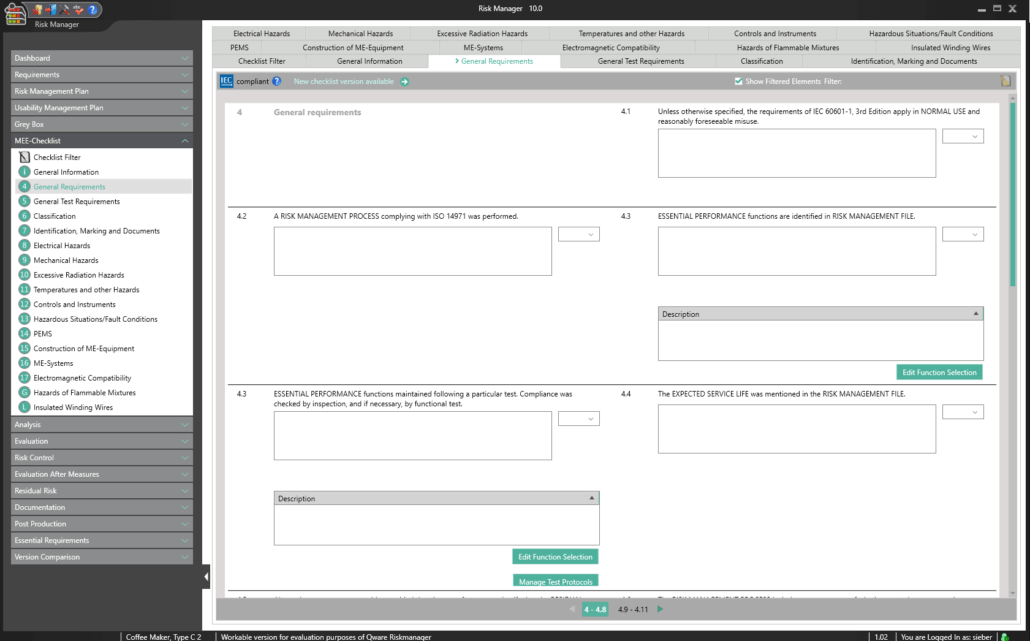
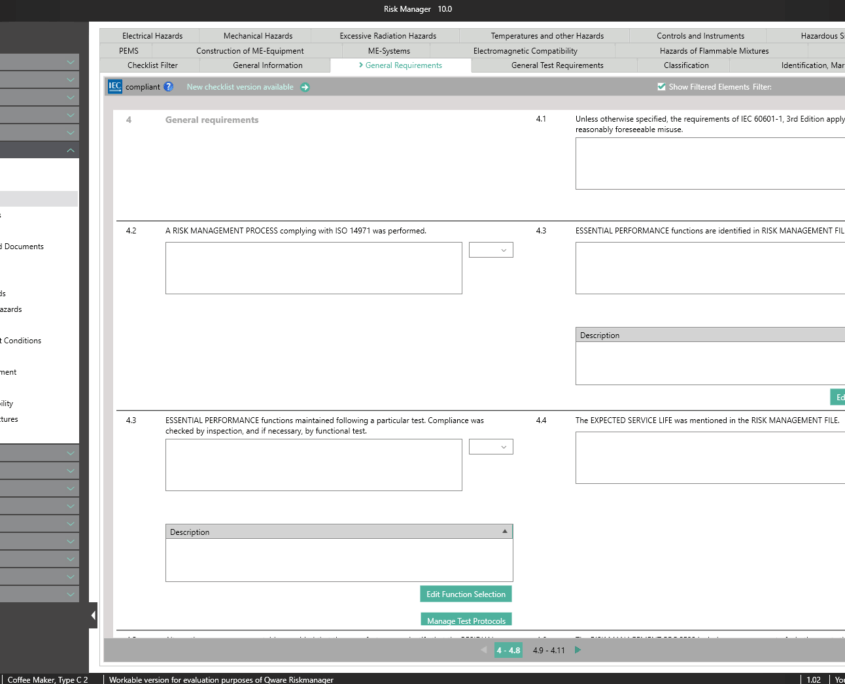
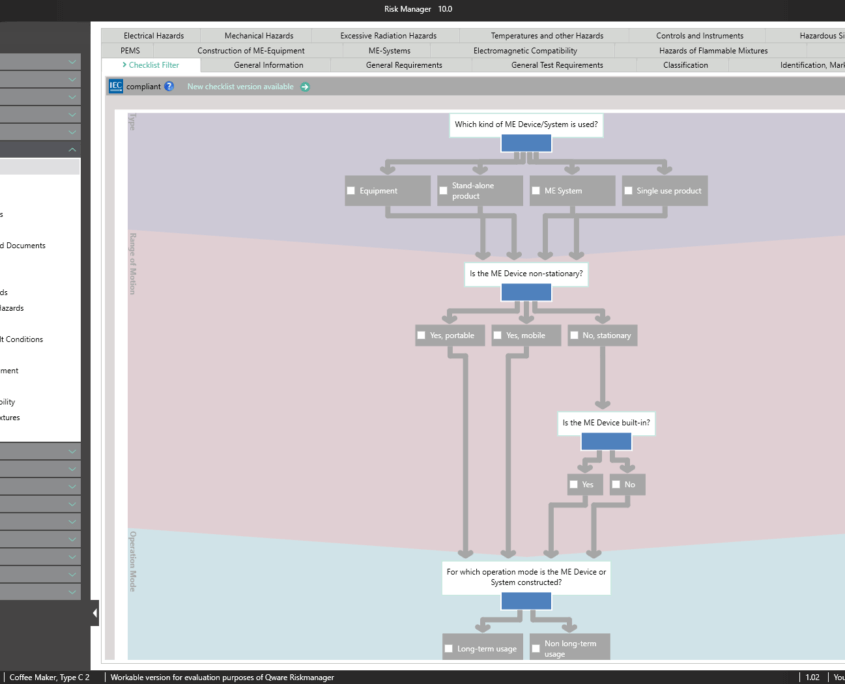
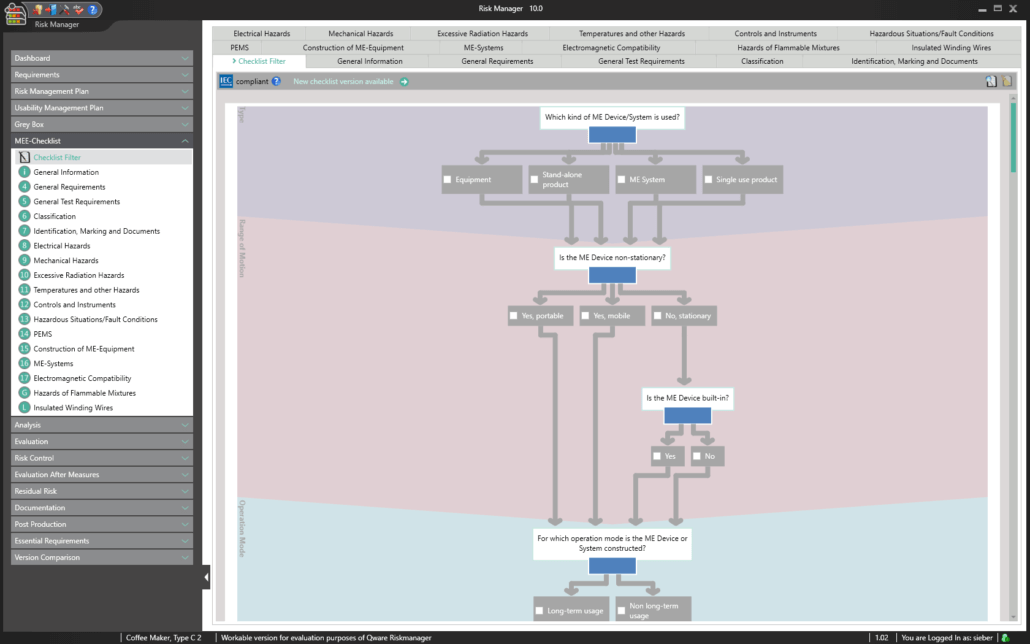
The Essential Requirements module of BAYOOSOFT Risk Manager enables you to accelerate the review and evaluation of these essential requirements significantly. With the help of an interactive checklist, the requirements are filtered according to your medical device, meaning you only need to answer the relevant questions. At the same time, the module is connected to risk management according to ISO 14971, meaning no redundant risk assessments are required to answer it. This enables you to benefit from time savings of up to 62% in reviewing the essential requirements, while you keep the technical documentation at the same assessment level consistently.
Conformity Report for Medical Electrical Equipment
IEC EN 60601-1 edition 3.1 – the key aspects
- Integration of the risk management process according to ISO 14971
- Consideration of more than 1,500 applicability tests
According to customer feedback, BAYOOSOFT Risk Manager accelerates this conformity assessment process pursuant to IEC 60601-1 by up to 62%.
This is made possible as non-relevant tests are automatically filtered out via intelligent querying. This way, you are interactively guided through the complete standard process, while the validated approval accelerator automatically answers all test questions not applicable to your medical device. The risk management process is also fully integrated, allowing you to connect to your existing assessments and analyses quickly and easily. Double collection is therefore a thing of the past.
Risk Manager is licensed by the IEC.
Machinery Directive
The Machinery Directive 2006/42/EC and the revised version 95/16/EC define a uniform level of protection to prevent accidents in the European Economic Area during the use of machinery. In addition, fundamental standards in health protection are defined.
With the extension module for the Machinery Directive, the BAYOOSOFT Risk Manager supports you in taking all requirements into account:
- With the help of the checklist-based documentation, you can easily and quickly record all requirements for a uniform level of protection for accident prevention.
- Using filters, you receive dynamically generated checklists and thus an overview of the requirements to be fulfilled. The checklist is structured analogously to the structure of the standard.
- The extension module also enables the complete integration of the risk management process according to ISO 14971, including the transfer of existing data from the analyses and assessments.
REQUIREMENTS ENGINEERING
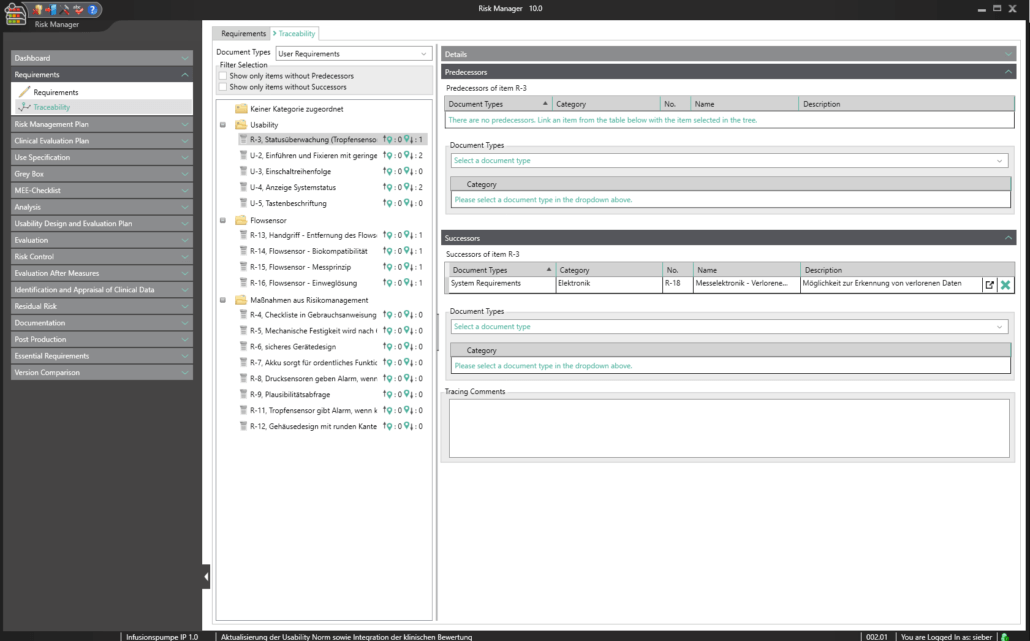
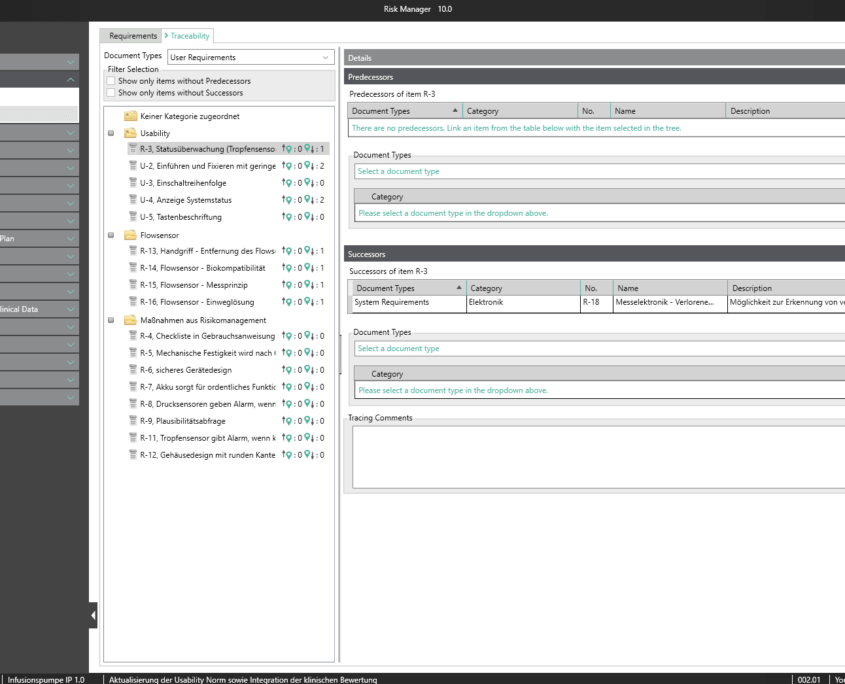
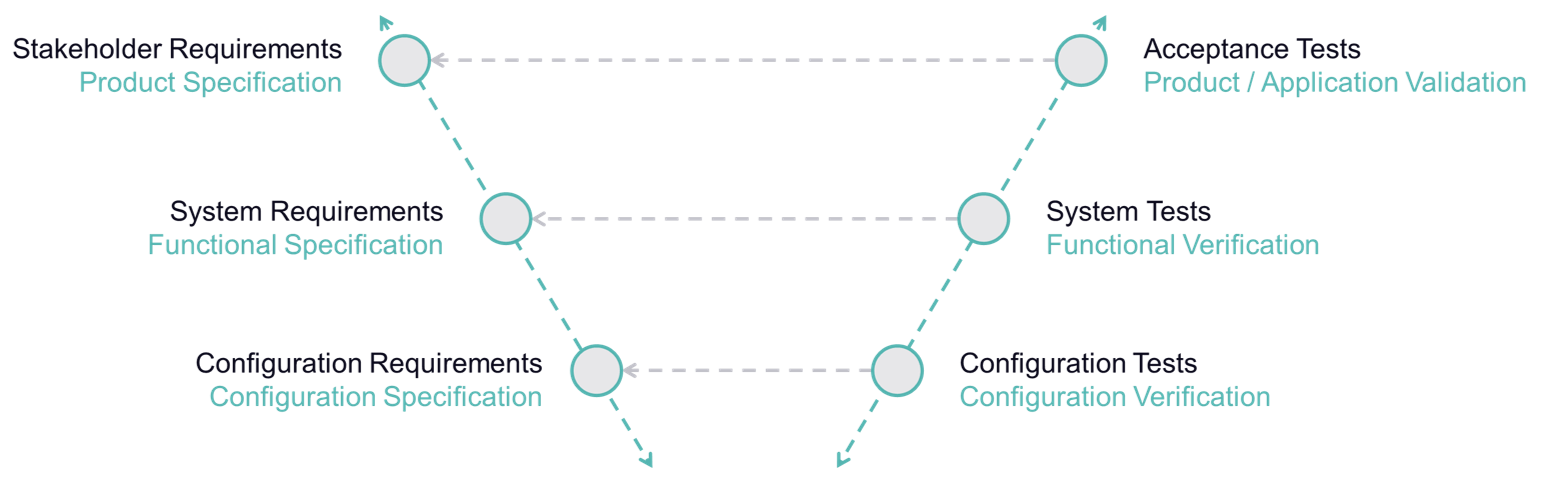
Requirements engineering is a key component in the efficient and low-error development of complex medical device systems and is particularly important for complex product and system concepts as well as shared development. Besides defining and implementing processes, this also covers the traceability of all specific requirements throughout the entire product lifecycle, as well as verifying/validating the requirements accordingly.
BAYOOSOFT Risk Manager fully integrates requirements engineering into the regulatory process that medical device manufacturers need to observe. New requirements can therefore automatically be derived from measures. What’s more, there is a direct connection between requirements (traceability across various documents), with resulting functions and validation and verification. Requirements relevant to usability are integrated into the usability engineering process, completely eliminating redundant data capture.
Clinical Evaluation
The medical device regulation MDR 2017/745 obliges you as a manufacturer of medical devices to perform a clinical evaluation throughout the entire lifecycle of a medical device. In addition to the systematic collection and analysis of pertinent clinical data from various sources, this encompasses a clinical follow-up of the medical device on the market.
The extension module of BAYOOSOFT Risk Manager enables you to generate clinical evaluations compliant with MDR and MEDDEV 2.7.1 rev4. They contain all relevant information for complying with the regulatory requirements. At the same time, complete coverage of the process as well as intuitive user guidance enables you to identify and check relevant data straight away.
1
2
3
4
5

1
Listing of all search results with current processing status
2
Integration of the Abstract
3
First assessment based on title and abstract
4
More detailed evaluation based on the full text
5
Conclusion of the evaluation
Software Lifecycle Process
Requirements of IEC 62304 on software lifecycle processes
1
2
3
1
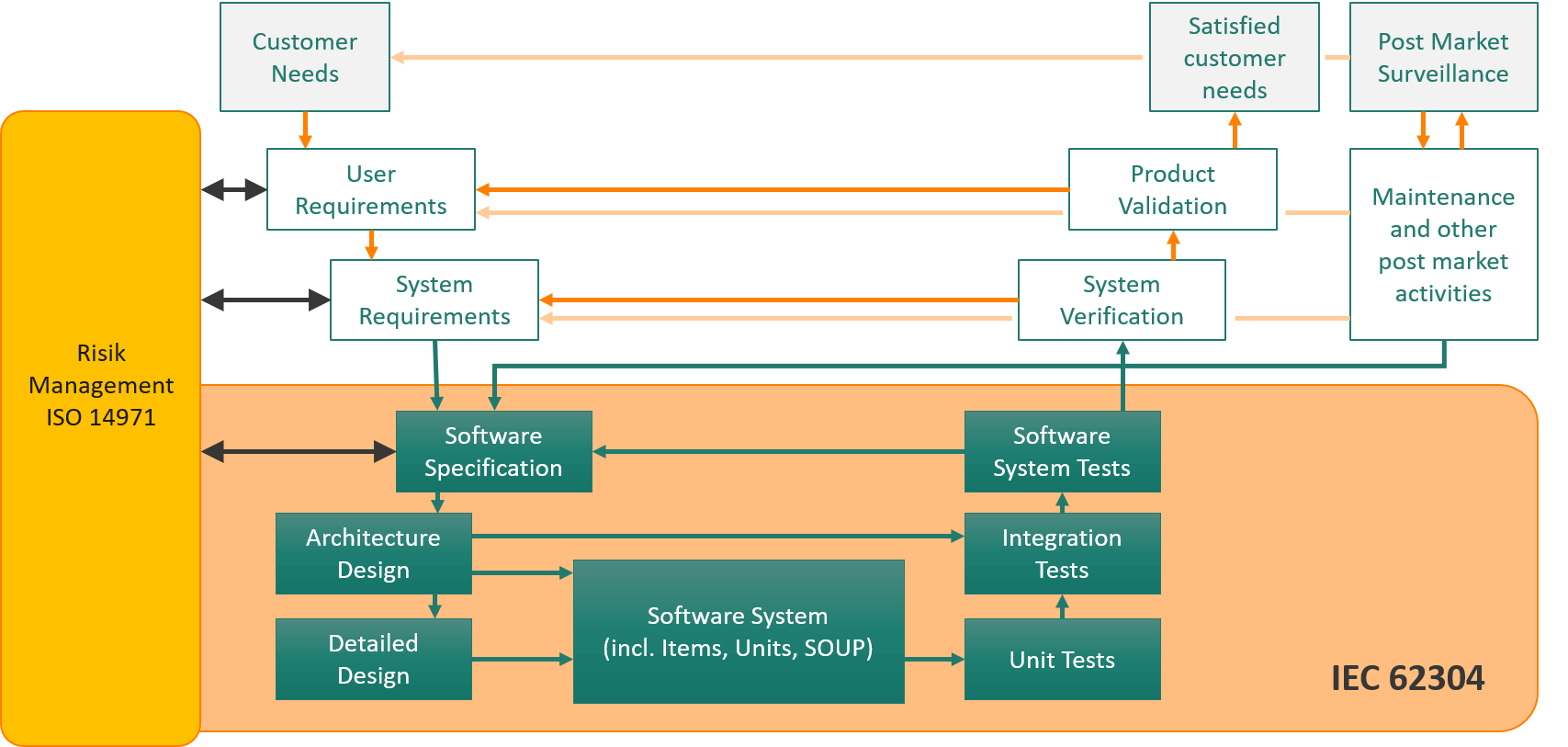
Clear requirements are legally necessary and at the same time essential for good products and efficient processes
2
IEC 62304 requires a risk analysis as an integral part of the development process:
- Software components must be considered in the hazardous situations
- Appropriate measures are to be implemented in the software
3
Proof of the conforming implementation of IEC 62304 can be provided by traceability.
IEC 62304 applies to the development and maintenance of medical device software if the software itself is a medical device or an embedded or integral component of a finished medical device. It stipulates that the medical device software must be developed and maintained within a risk management system. This means, for example, that software requirements must be considered as part of risk analysis. At the same time, it is important to ensure that effective measures are considered in the software requirements.
Traceability matrices can be used as proof that the medical device software has been developed in compliance with IEC 62304. These make sure that customer requirements are checked as part of suitable validation processes and that they are integrated into the software and verified against the requirements. Especially for large systems, this can be a challenging task.
BAYOOSOFT Risk Manager offers a solution for preparing technical documentation in compliance with IEC 62304 for developing your medical device software. Here, the two extension modules Requirements Engineering and REST API are combined with risk management according to ISO 14971:
Seamless integration of requirements management into risk management enables you to replace redundant entries and manual copying with just a few clicks in the system. Connect requirements with functions in risk management as well as requirements at a further level (traceability) or quickly and easily create new requirements based on the effective measures.
Create test plans for validating and verifying the requirements and document the respective test runs. Test cases are linked directly with requirements, also allowing you to ensure permanent traceability here.
Ensure all requirements are linked with user stories in source code management, for example. The REST API opens up the further system environment for you in this connection. Integrate your source code management or ticket system for post-market surveillance – also enabling you to directly link with elements at other levels.
Finally, simply generate your traceability matrices at the press of a button based on the auditable and centrally stored, fine-grained and dynamically interconnected information. Moreover, the integrated version management of BAYOOSOFT Risk Manager enables you to “close” certain version statuses, so that they can no longer be changed retroactively.
Which systems should you link for IEC 62304-compliant software development?
REST API
BAYOOSOFT Risk Manager offers a REST API as an extension for integration into existing infrastructure. If new insights emerge from ticket systems that need to be considered in market monitoring or if processes in your document management system require a new version of risk management files, these can be fully automatically transferred to BAYOOSOFT Risk Manager via the web interface and processed, for example.
Pre-Validation Package
All systems involved in the process need to be validated in accordance with DIN EN ISO 13485:2017. BAYOOSOFT Risk Manager has a validation process based on the GAMP 5 guidelines for computerized validation according to these requirements and is validated as category-four software. The following aspects were considered in this respect:
The Pre-Validation Package contains the following documents:
Validation Plan
User Requirements Specification
Supplier Audit Questionnaire
Risk Assessment
Functional Specification
Installation Guide
Installation Qualification
And many more