BAYOOSOFT Risk Manager
Core features for faultless technical documentation
BAYOOSOFT Risk Manager is the validated approval accelerator for preparing technical documentation for manufacturers of medical devices and in-vitro diagnostics. All relevant information is recorded in a clearly structured environment, stored in a central, audit-proof manner and dynamically connected with each other at a fine-grained level. No redundant data exists. Reports are automatically generated with up-to-date data at the press of a button.
The intuitive user guidance, examples provided and ready-made document templates make it easy to get started. The self-learning knowledge database results in synergy effects between projects and helps to utilize developed know-how throughout the company. At the same time, BAYOOSOFT Risk Manager meets the requirements of FDA 21 CFR Part 11.
A clear and consistent approach with the reliable application accelerates market approval and has a positive effect on expended time and costs: Our customer base of more than 800 customers reported up to 62% faster approvals for medical devices thanks to BAYOOSOFT Risk Manager.
These key features provide the foundations for conveniently generating technical documentation. Find out more:
Structured Work According to Lifecycle Phase
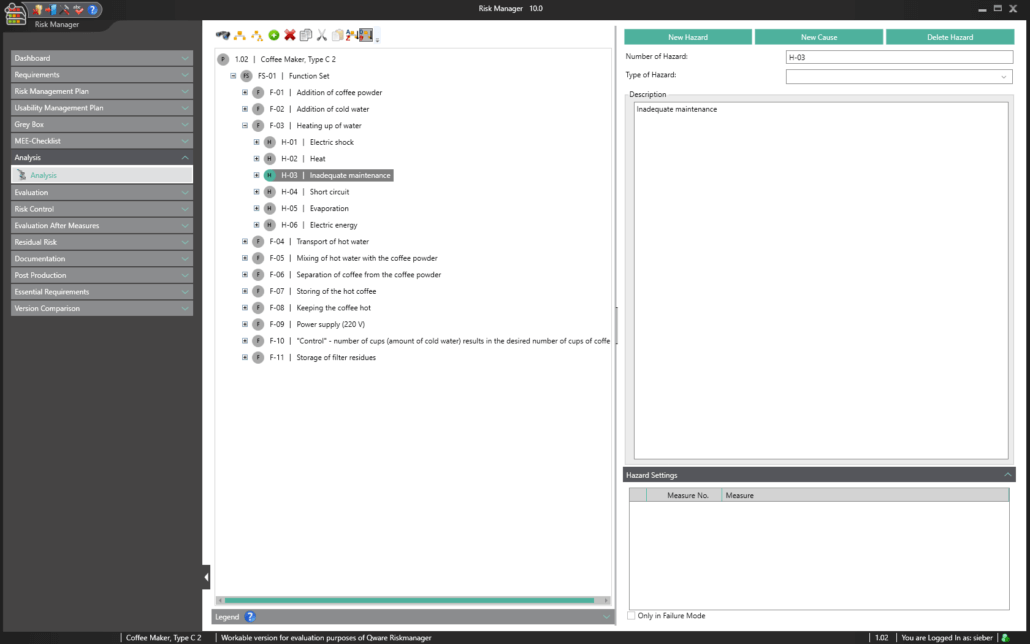
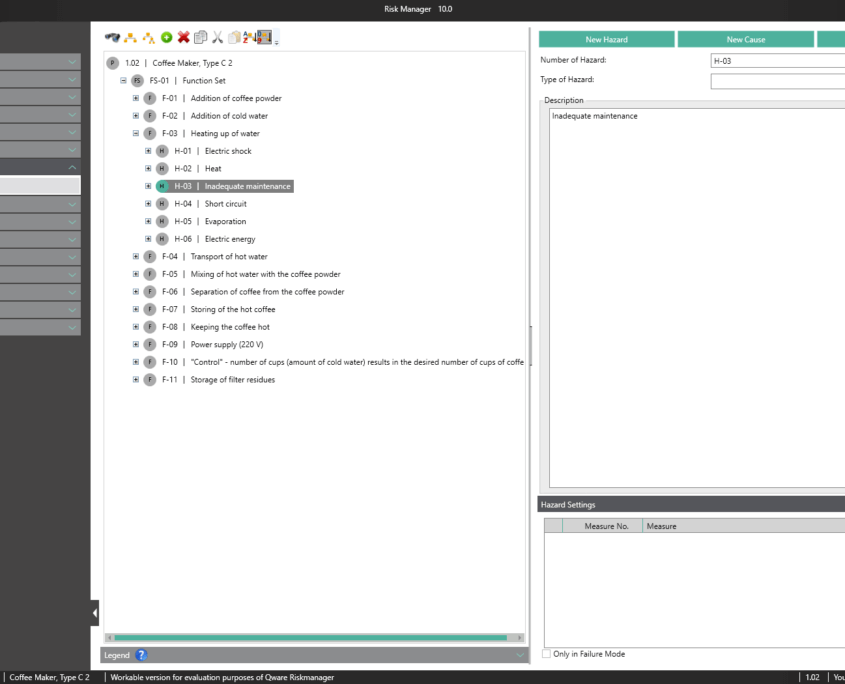
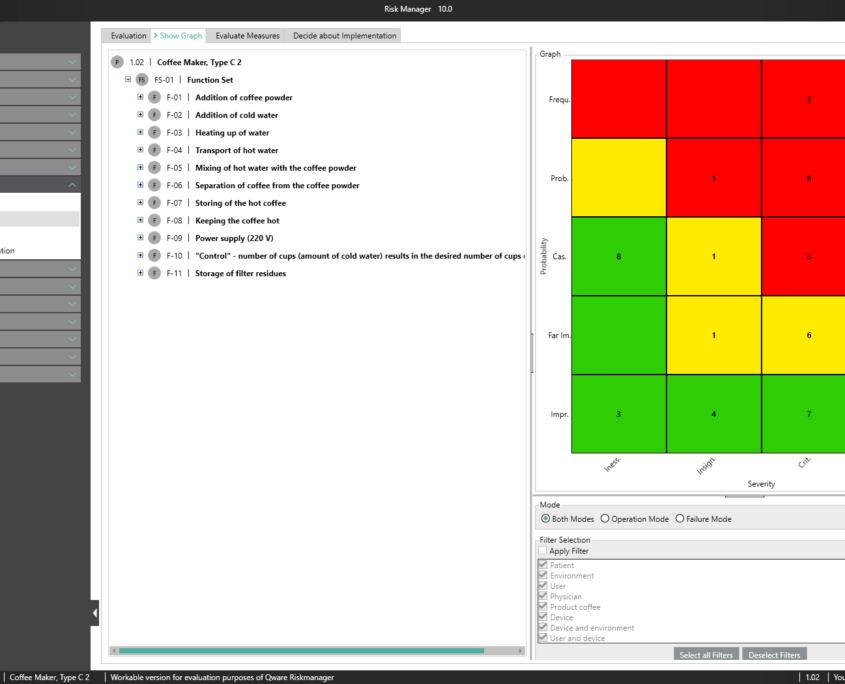
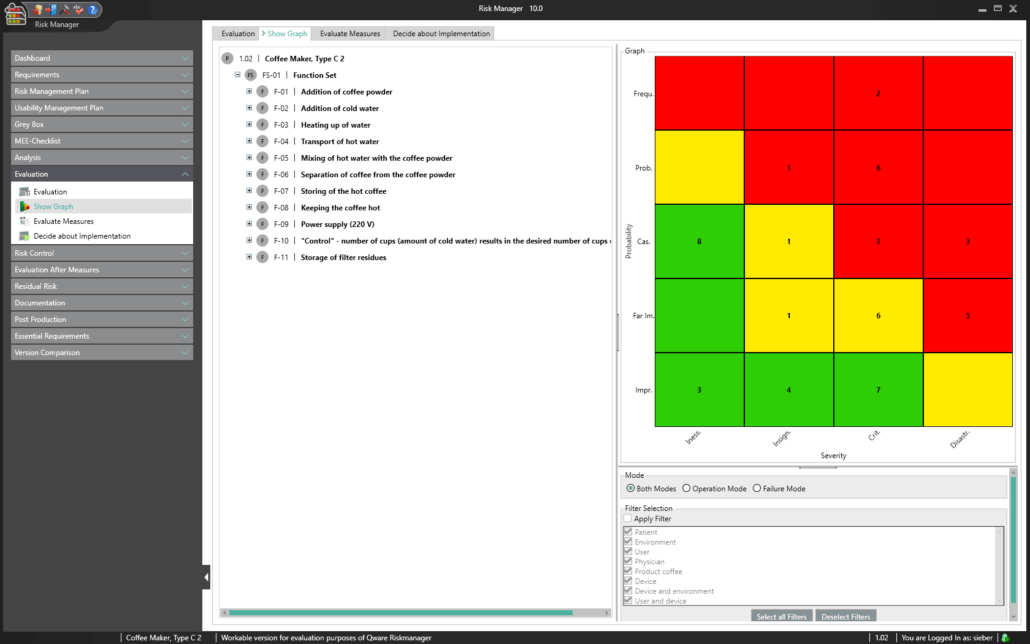
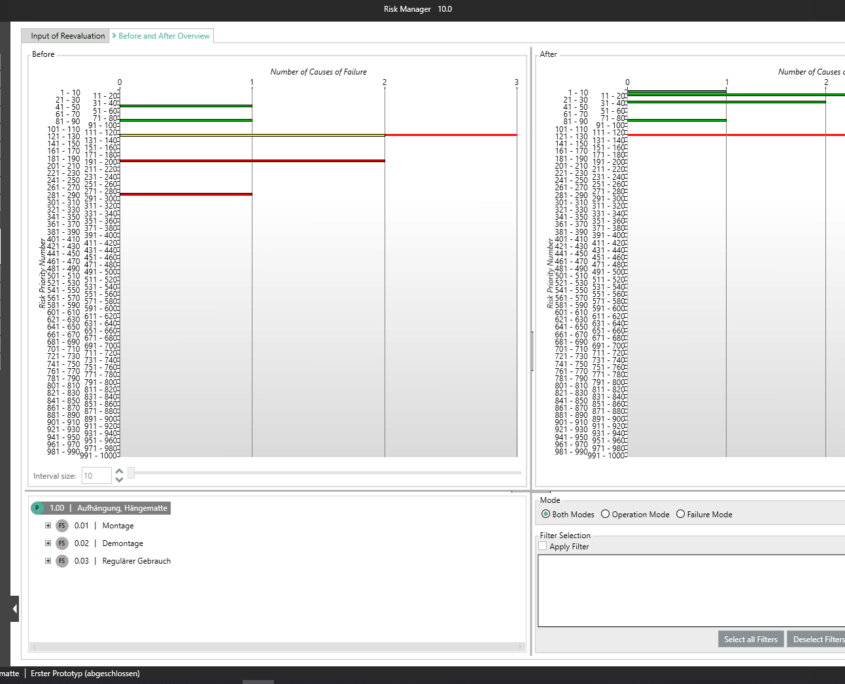
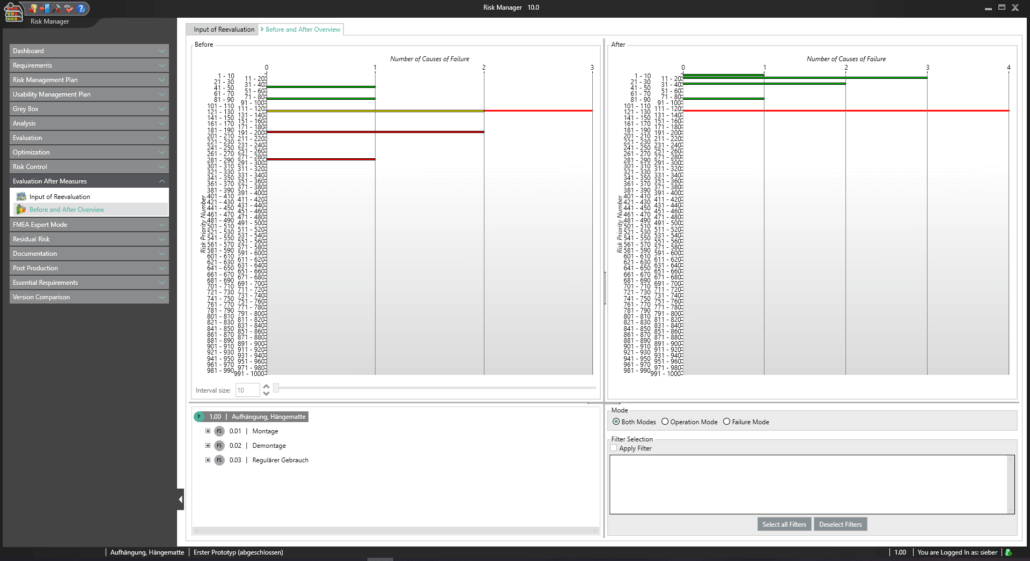
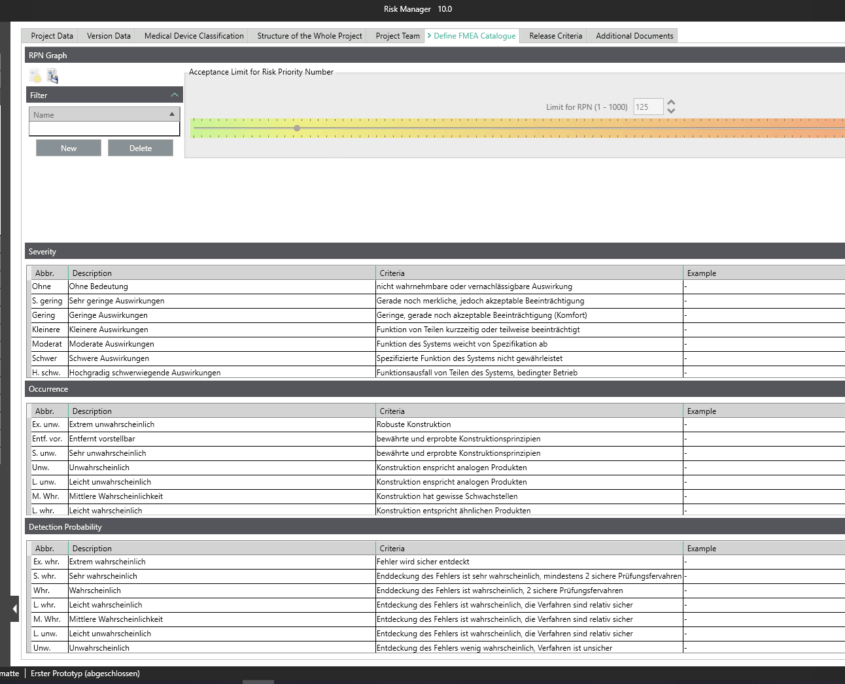
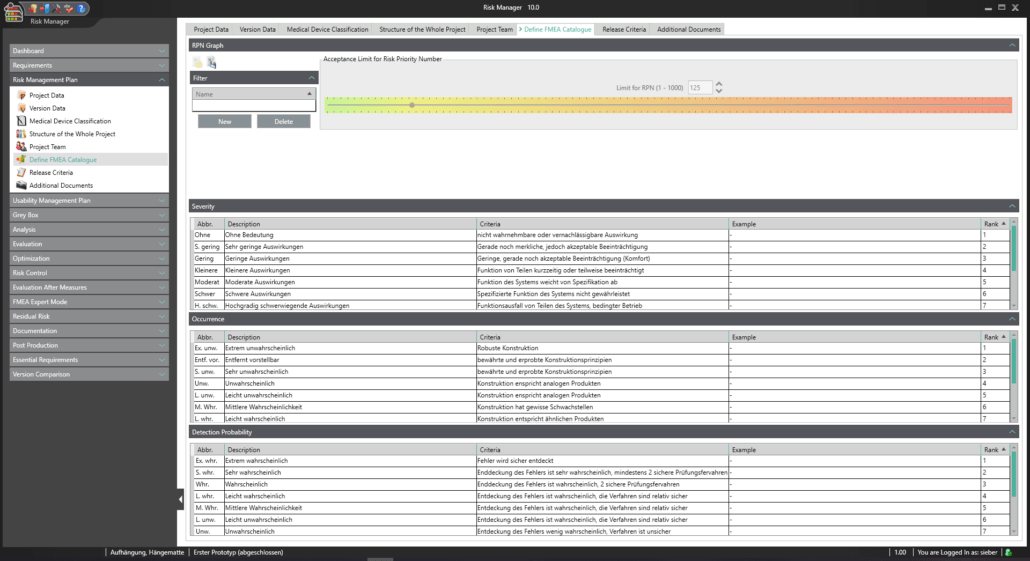
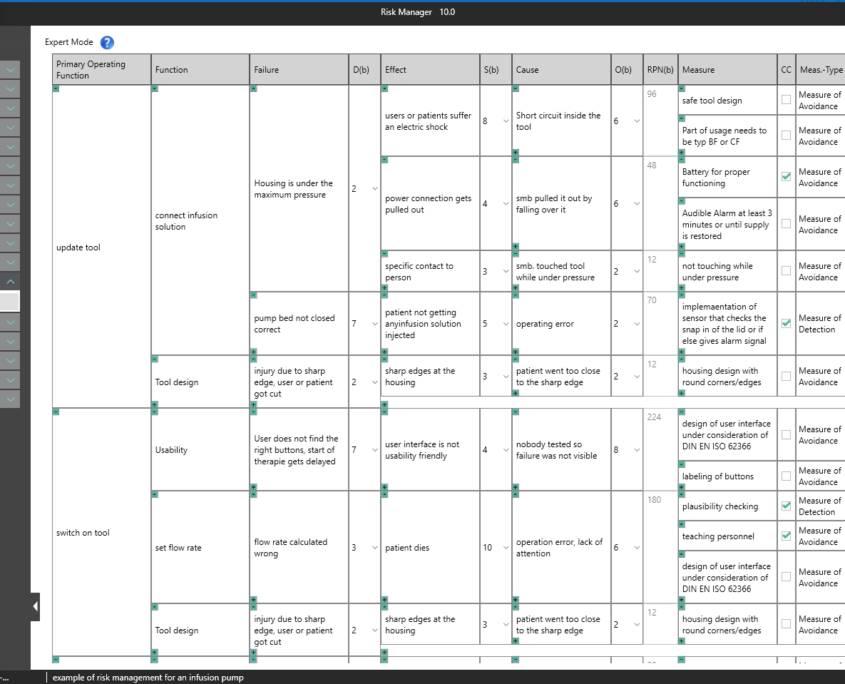
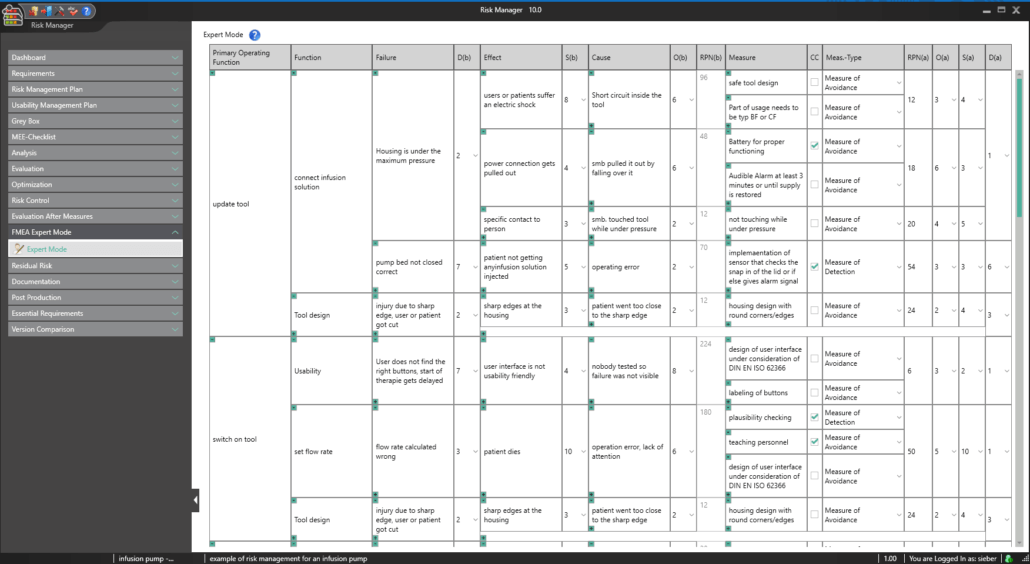
BAYOOSOFT Risk Manager offers you a project and version-based approach for managing your technical documentation. You create projects for your medical devices and can then historicize changes to your risk assessment as well as other module features in versions, depending on the device lifecycle phase or other user-defined steps. By “closing” various versions, you can ensure that certain versions can no longer be changed retroactively. You then work on subsequent versions.
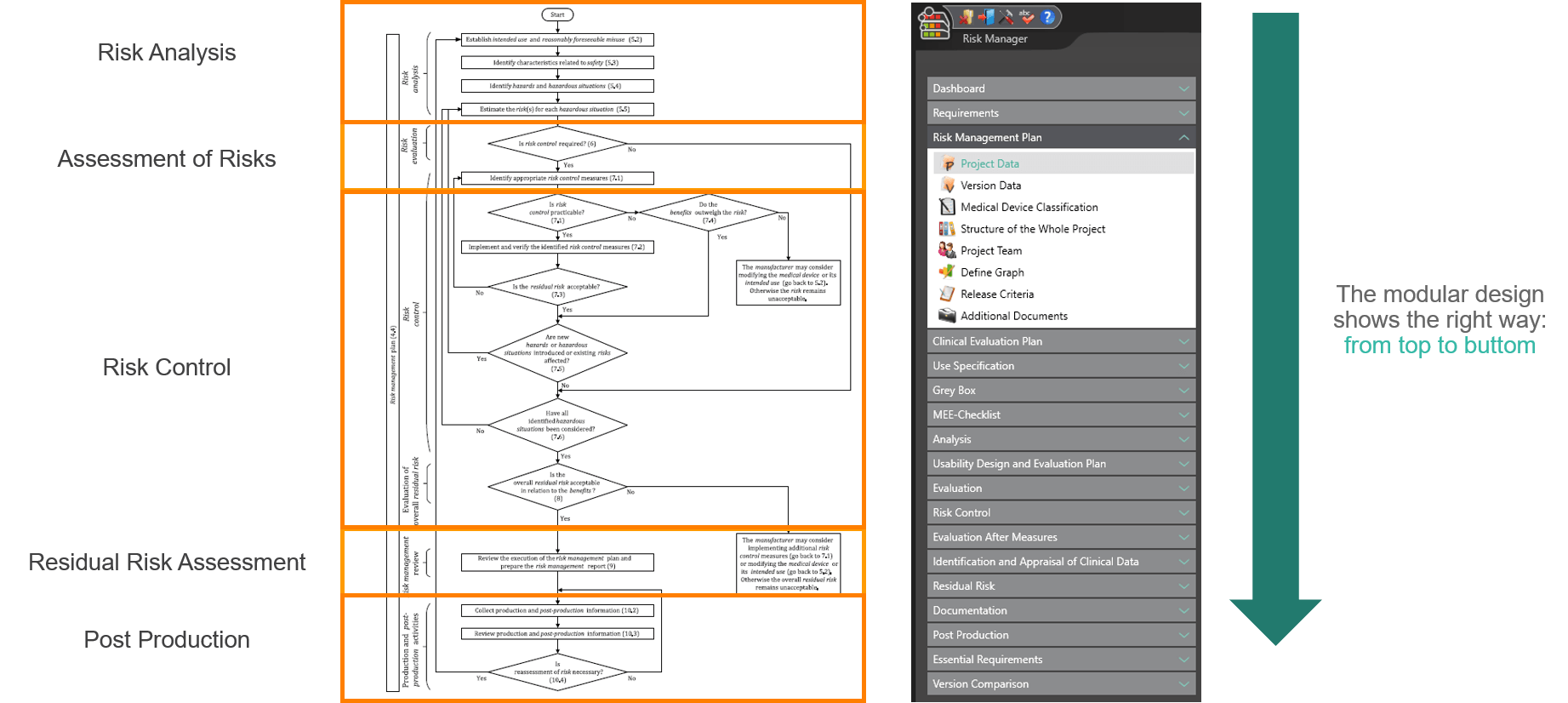
The process for preparing risk management documents is structured in accordance with the essential activities of the risk management process as required by the standard. The following processes are distinguished:
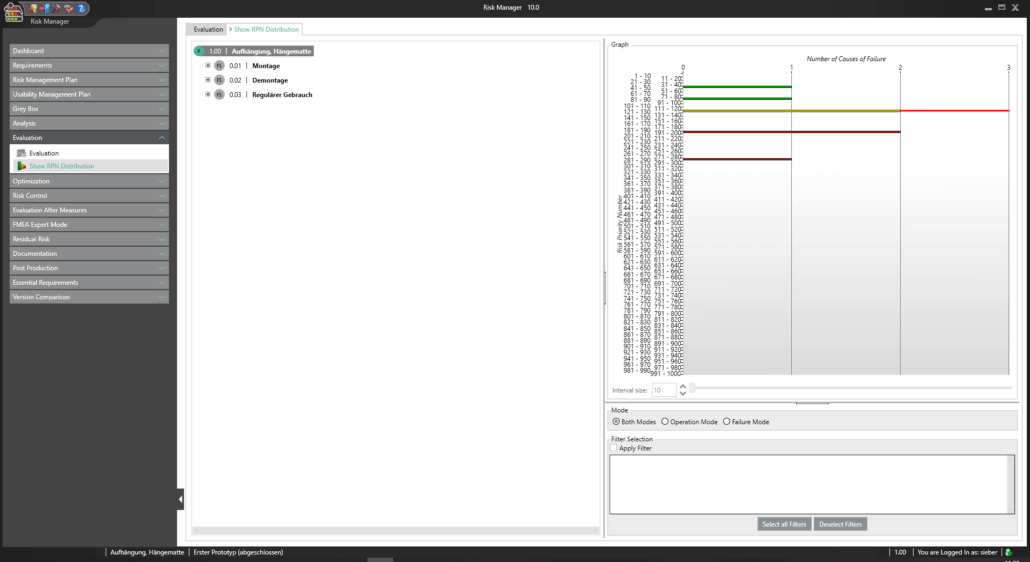
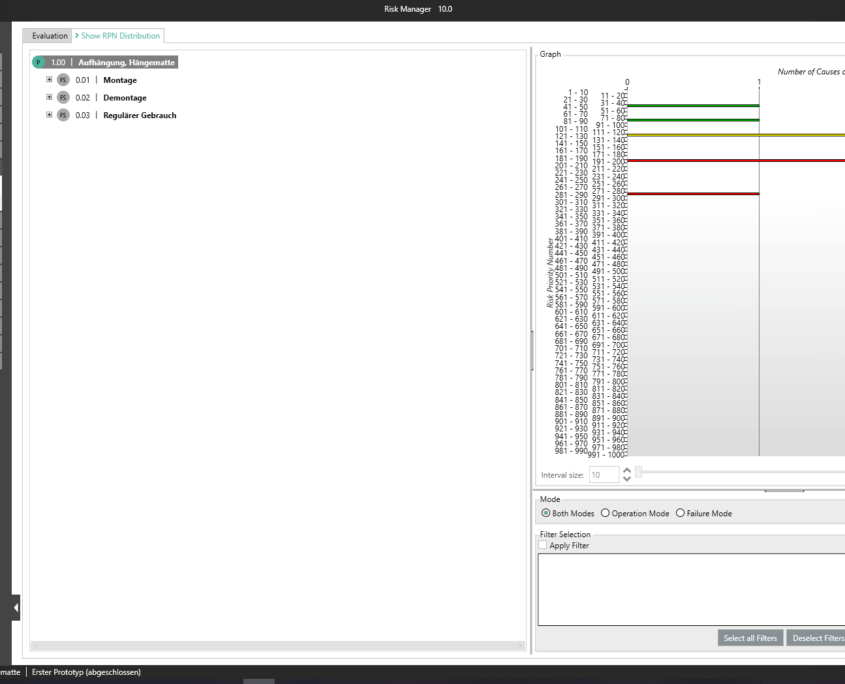
This way, you transparently record the contents and results of your risk management activities and are guided through the process in a structured manner. The automatically generated documents contain your extensive information on your risk management file. The structure of BAYOOSOFT Risk Manager helps you work purposefully and efficiently as you follow the process from top to bottom in the menu tree. BAYOOSOFT Risk Manager thereby accelerates your development and approval process – allowing you to dedicate your time to the content not the process.
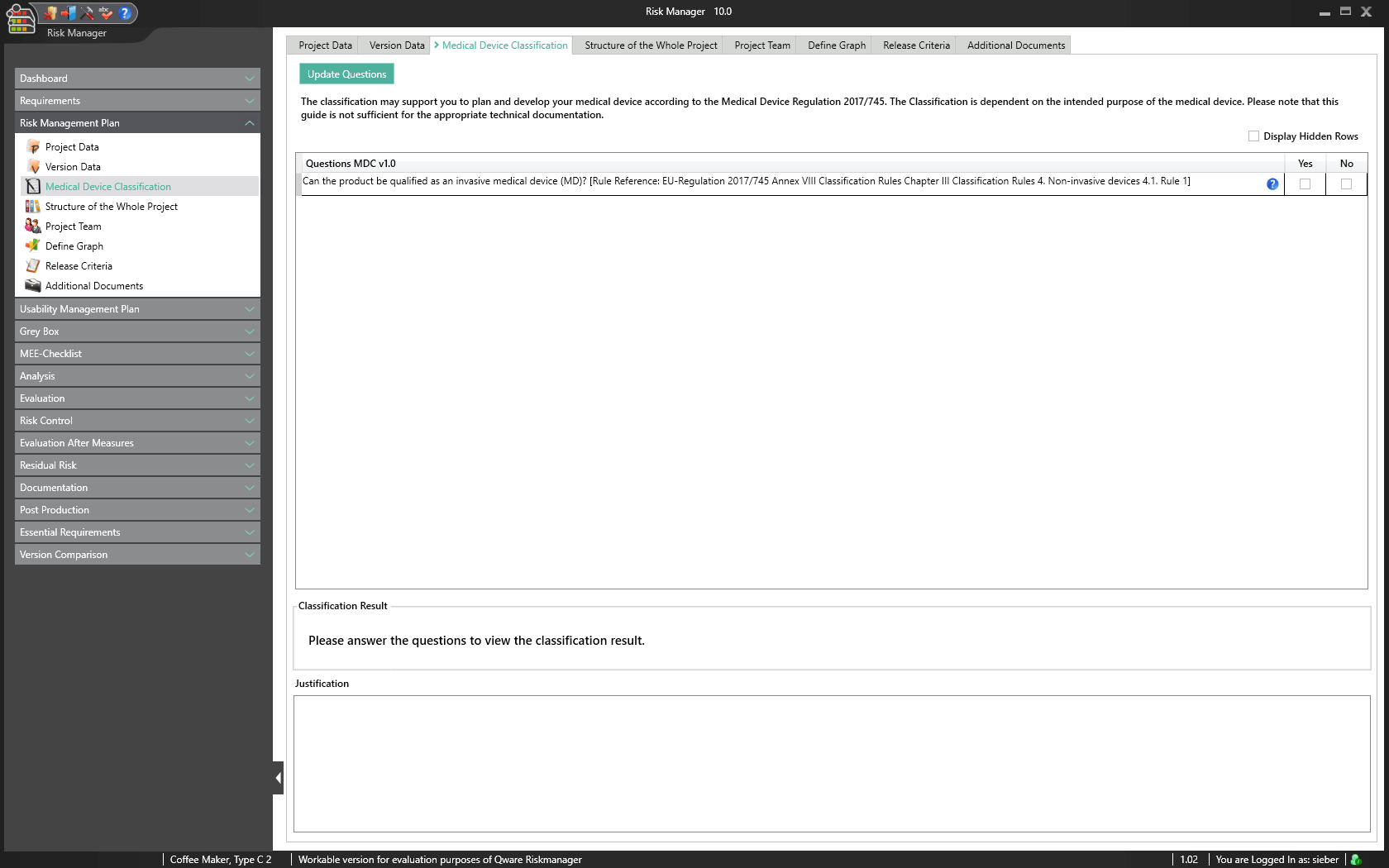
Medical Device Classification
Technical documentation is a requirement for the registration or approval of your medical device. Here, the complexity and type of device determines the risk classification and the scope of necessary documents.
To provide optimal support in classification according to the medical device regulation (MDR Article 51 (1)), we have fully integrated the functionality for classifying the medical device into categories I, IIa, IIb or III in the validated approval accelerator. Based on an interactive questionnaire, you are guided through the classification process and can issue the result as a report. This provides you with an initial reference for classifying your medical device. You then only need to give a justification for the classification, considering the intended purpose and stating the applied rules.
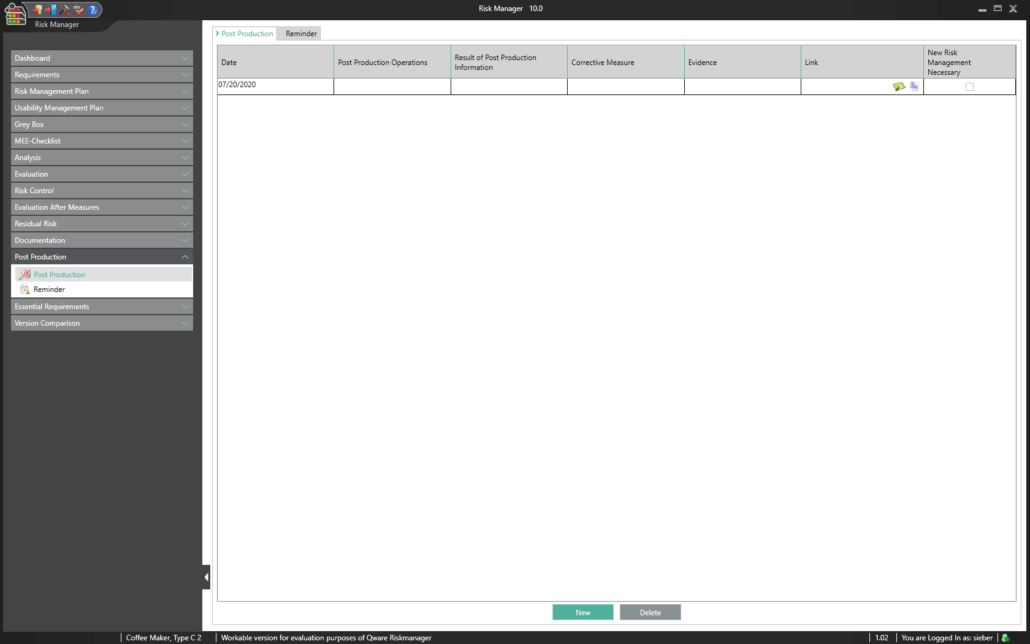
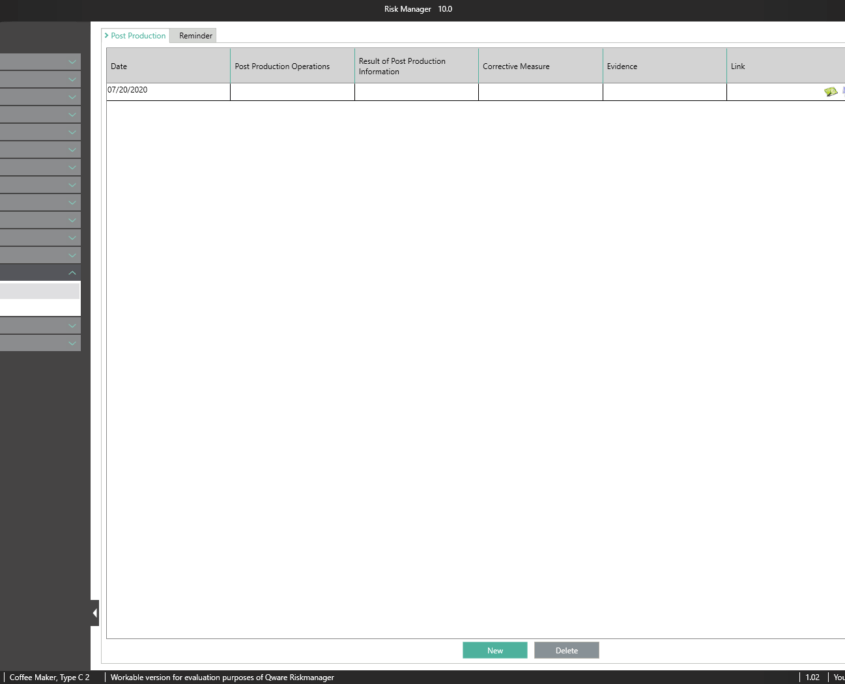
Post Market Surveillance
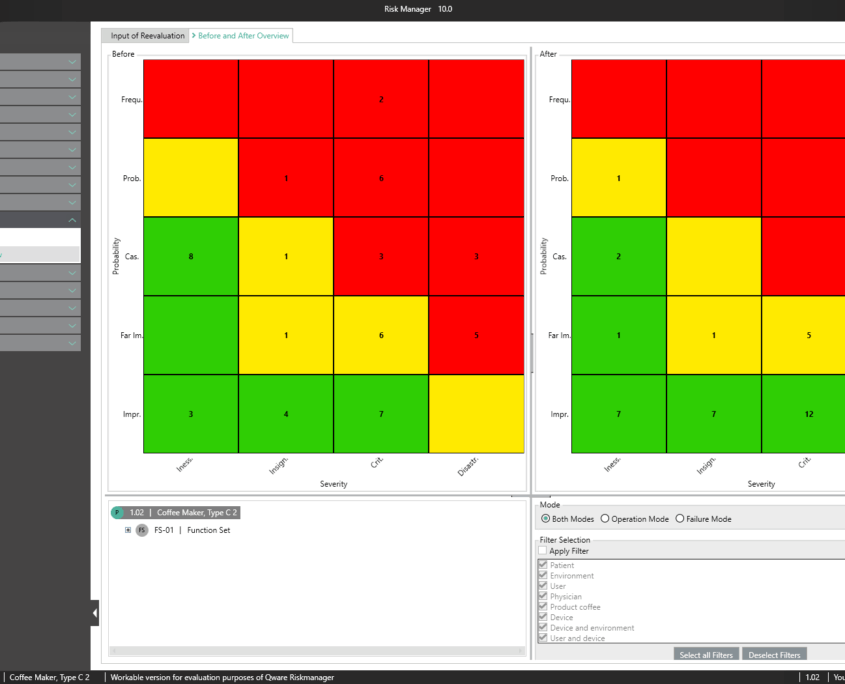
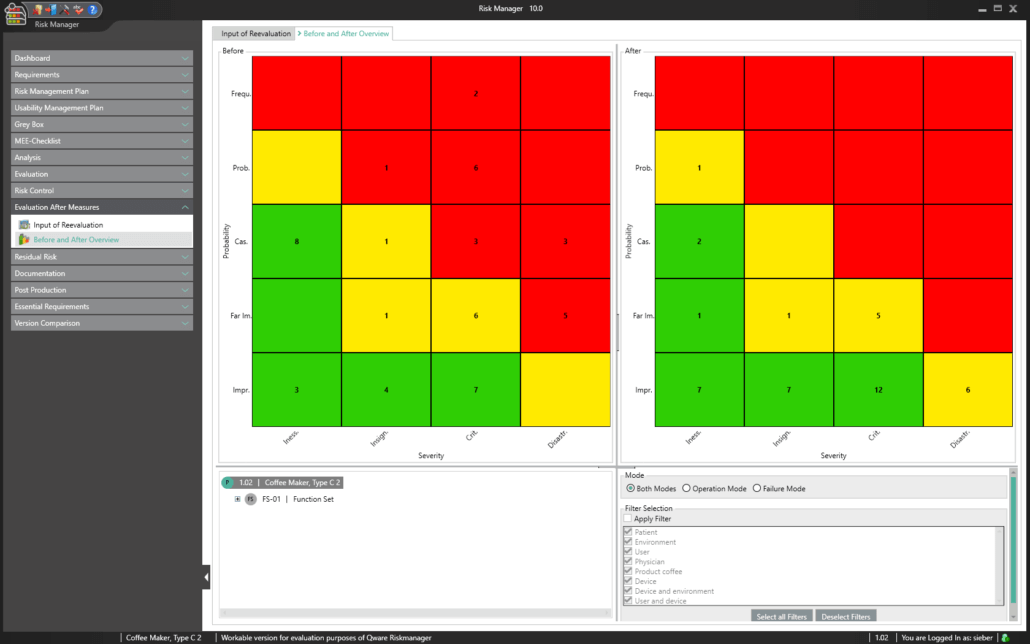
Post-market surveillance refers to a proactive and systematic process for identifying information about medical devices already brought into circulation and for deriving necessary corrective and preventive measures.
As a manufacturer of medical devices, you are required to define such a systematic process in order to record information about your medical devices or similar products in the lifecycle phases following production. The information collected can be assessed in relation to its potential safety relevance, before defining appropriate measures for minimizing risks associated with your medical device. The aim of the ongoing process is to ensure that your medical devices offer patients the benefits promised and that no unmanageable risks exist.
Thanks to the market monitoring section included in the basic module, BAYOOSOFT Risk Manager gives you the ability to document your observations. Gathered as part of a ticket system or other system, they can automatically be transferred to BAYOOSOFT Risk Manager via the REST API interface as an extension module for integration into your infrastructure. If during post-market surveillance you determine that a new risk assessment is necessary, this can be suitably proven and documented in this report.
Self-Learning Knowledge Database
The intuitive structure of BAYOOSOFT Risk Manager guides you through the process of risk management (in the menu system, from top to bottom). The knowledge developed grows across projects in the self-learning knowledge database. The information is made available to authorized employees throughout the company. Here, the knowledge database supports your work by providing frequently recurring elements for preparing a new risk analysis. Groups are used as suggestion lists for new elements in the risk analysis.
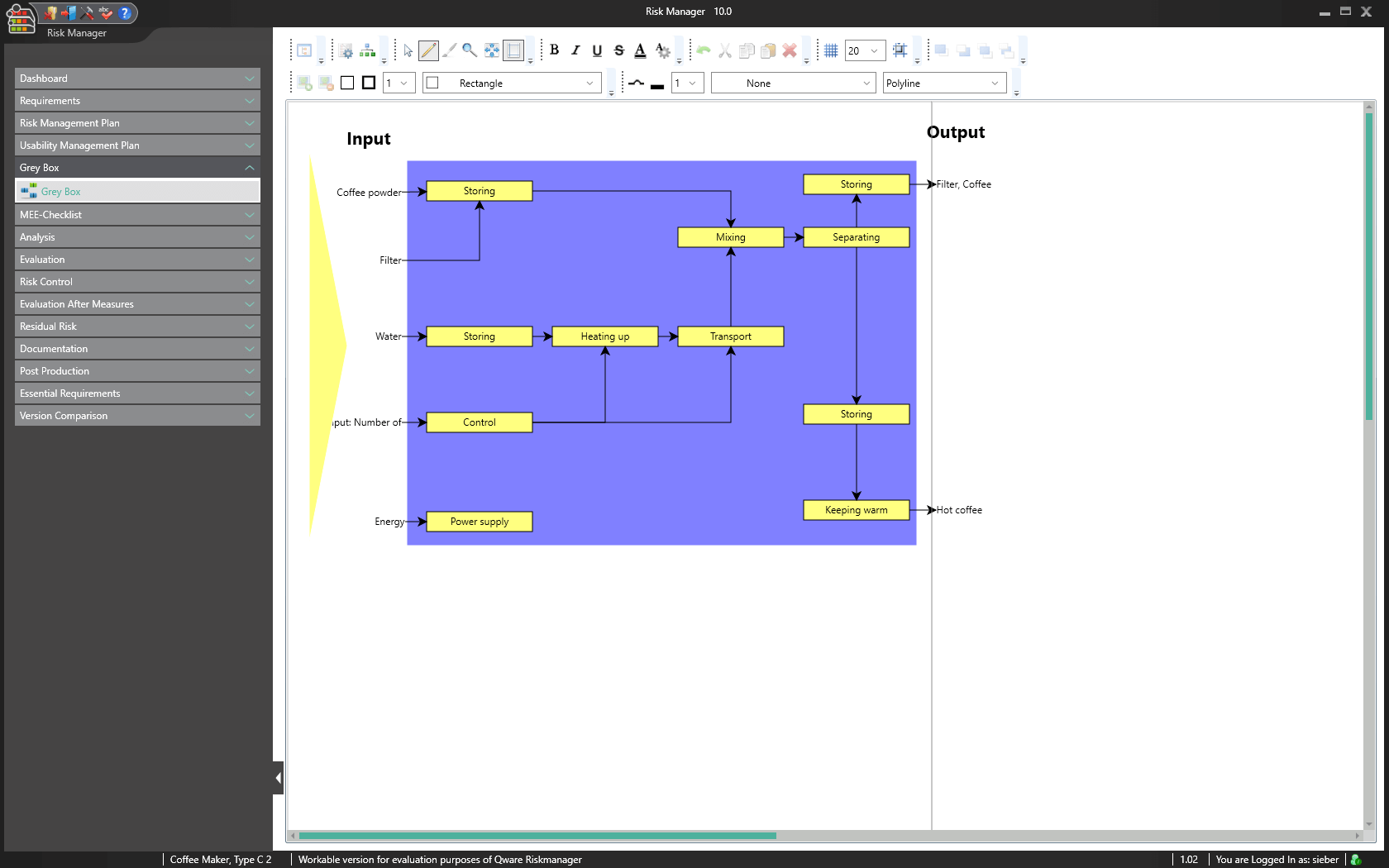
Visualizing with the Grey Box
The BAYOOSOFT Risk Manager basic module includes the grey box, offering the function of an electronic whiteboard. Use it to create process plans, device designs or flow charts, for example. The intuitive graphical editor lets you integrate function dependencies, disturbance variables, inputs and outputs, helping you quickly identify risks and select suitable measures for reducing risk.