Meeting the requirements with the BAYOOSOFT RISK Manager
Clinical evaluation of medical devices according to MDR
According to the Medical Device Directive MDR 2017/745 and MEDDEV 2.7.1, manufacturers are obliged to conduct a clinical evaluation over the entire life cycle of a medical device. This serves the following objectives:
Sources of clinical evaluation
According to MDR Article 2(48), clinical data refers to information obtained in the course of use concerning the safety and performance of the device. Such data may be generated in a clinical trial or taken from the specialist literature. This includes, inter alia, clinical trials already conducted or other studies published in the specialist literature concerning a device that has been proven to be similar.
Clinical evaluation of medical devices in the Risk Manager
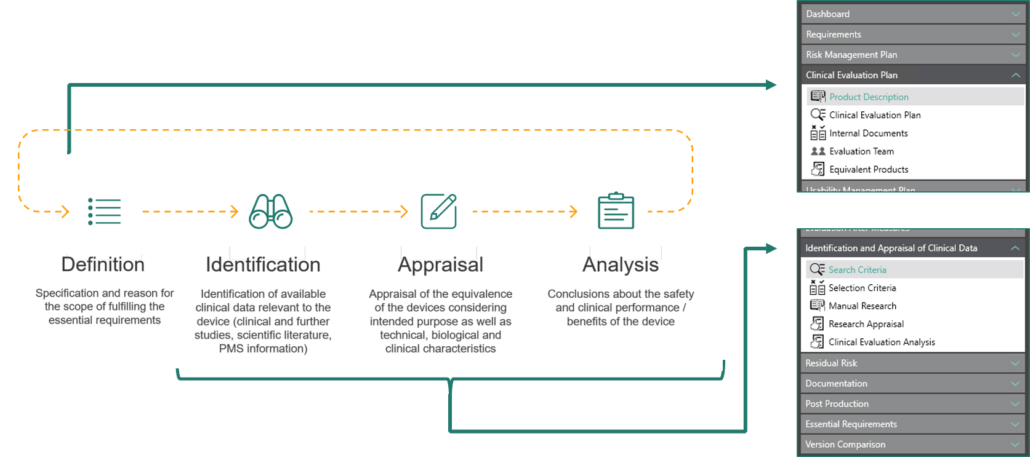
The “Clinical Evaluation” module of the BAYOOSOFT Risk Manager offers a solution compliant with MDR and MEDDEV 2.7.1 rev4 to support you in the same process. An intuitive user interface as well as the complete coverage of the process phases allow you to start immediately with the identification and verification of the relevant data.
In the “Clinical Evaluation Plan” section, you describe your product in text form, add images and plan the scope of the clinical evaluation taking into account the state of the art, claims and features. You can add references to existing studies and internal documents and evaluate the similarity of equivalent products.
Identify and evaluate clinical data relevant to the device and its intended use through a systematic scientific literature search. Appropriate search and selection criteria define the search. Subsequently, the search results are evaluated in several steps, from selection based on title to the assessment of safety, performance and clinical benefit of the own medical device.